Overview
Amendments are created for approved or suspended protocols when any part of the protocol requires a change. Depending on the type of change, the amendment may or may not need to go through the full review process.
Important Information
Personnel, Scientific, and PI Change amendments require updates to Animal Handling Details.
There are four types of amendments, allowing multiple amendments to be processed simultaneously depending on the type. Protocols can have one each of Personnel, Funding, and Scientific amendments open at a given time.
- Personnel Amendment – Unlocks the non-PI Personnel page for changes, such as adding or removing lab staff listed on the protocol, or updating permissions for lab staff. Requires administrative review for approval.
- Funding Amendment – Unlocks the Funding Sources page for changes to the external and internal funding sources. Requires administrative review for approval.
- Scientific Amendment – Unlocks the species and procedures pages for changes, such as goals, protocol description, or animal justification. Requires committee review.
- Principal Investigator (PI) Amendment – Unlocks the full protocol for changes, such as changing the PI to another valid PI. Requires committee review.
To create a Principal Investigator (PI) amendment, no other amendments can be open on the protocol, and no additional amendments can be added while the PI amendment is open.
Only personnel with edit rights on the protocol can start an amendment. The PI is the only person who can submit an amendment for review.
Step-by-Step Process
Personnel Amendment (Add, Update or Delete Personnel)
- From the Protocol workspace, click the Create Personnel Amendment button.
- Click Finish.
Note If you do not want to create a Personnel amendment, click Exit (close without Saving) to return to the protocol page. - Click Edit Amendment.
- Make changes to the personnel information by changing answers, clicking Add, Update, or
 (Delete) as needed.
(Delete) as needed.
Note See Add Personnel for detailed steps on working with personnel data.
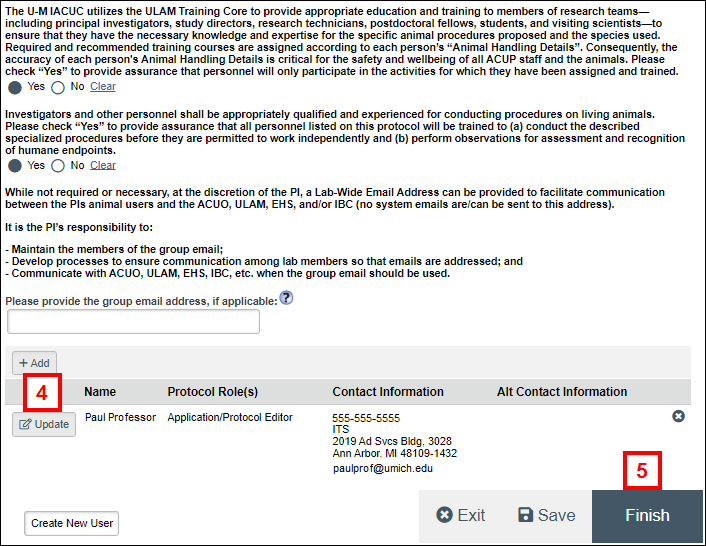
- Click Finish.
- Click Submit Amendment under Activities.
Notes- Error checking is performed on the amendment when it is submitted. If any errors are found, a list displays. All errors must be resolved before the amendment can successfully be submitted.
- See Add Animal Handling Details for steps on working with animal handling details.
- Click OK.
Funding Amendment (Add, Update or Delete Sponsor/Funding)
- From the Protocol workspace, click the Create Funding Amendment button.
- Click Finish.
Note If you do not want to create a Funding amendment, click Exit to return to the protocol page. - Click Edit Amendment.
- Make changes to the External Sponsor/Funding Sources information by changing answers, clicking Add, Update, or
 (Delete) as needed.
(Delete) as needed.
Note See Add Sponsor/Funding Information for steps on working with sponsor/funding data.
- Click Finish.
- Click Submit Amendment under Activities.
Note Error checking is performed on the amendment when it is submitted. If any errors are found, a list displays. All errors must be resolved before the amendment can successfully be submitted. - Click OK.
Scientific Amendment
- From the Protocol workspace, click the Create Scientific Amendment button.
- Click Finish.
Note If you do not want to create a Scientific amendment, click Exit to return to the protocol page. - Click Edit Amendment.
Note A scientific amendment allows the data on the following pages to be changed: Protocol Scope and Relevance, Species, Activities, Pain or Distress, Location(s) and Transportation, Environment, Procedure Selection, Procedures, Agent/Substance Summary. - Click Continue or use the Navigation menu to go to the applicable scientific page.
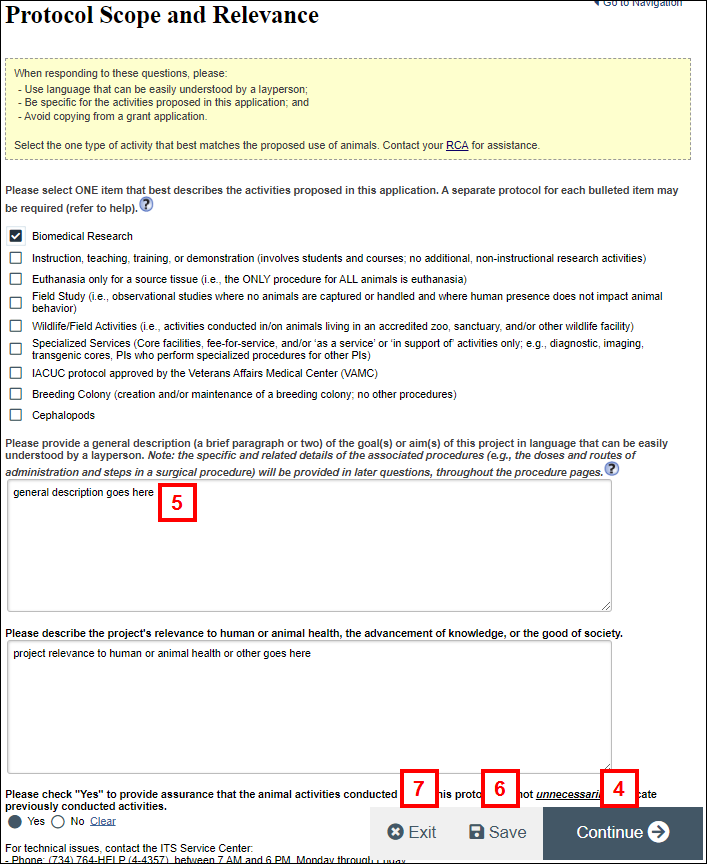
- Make all necessary changes on the page.
Notes See the following procedures for detailed steps on working with scientific data:
- Repeat steps 4-5 until all scientific changes have been made.
- Click Save.
- Click Exit.
Note Click Save Changes & Exit if a dialog box displays. - Click Submit Amendment under Activities.
Notes
- Error checking is performed on the amendment when it is submitted. If any errors are found, a list displays. All errors must be resolved before the amendment can successfully be submitted.
- See Add Animal Handling Details for steps on working with animal handling details.
- Click OK.
Change PI Amendment (Add, Update or Delete PI)
- From the Protocol workspace, click the Change PI button.
- Click OK.
Note If you do not want to create a PI amendment, click Exit to return to the protocol page. - Click Edit Amendment.
Note A PI amendment allows the data on all application pages to be changed. - Click Continue or use the Navigation menu to go to the applicable application page.
Notes See the following procedures for detailed steps on working with application data:
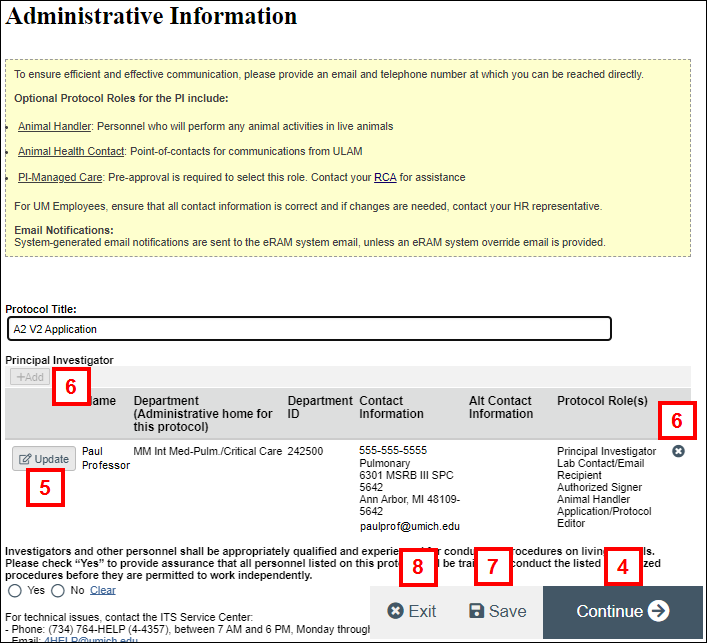
- If you wish to update details for the listed PI, click Update.
- If you wish to replace the PI, you first must click
 (Delete) to remove the listed PI, and then click Add to add a new PI.
(Delete) to remove the listed PI, and then click Add to add a new PI.
Note See the Add a Principal Investigator procedure for detailed instructions. - Click Save on each page when you have finished making changes.
- Once changes to all pages are complete, click Exit.
Note Click Save Changes & Exit if a dialog box displays. - Click Submit Amendment under Activities.
Notes
- Error checking is performed on the amendment when it is submitted. If any errors are found, a list displays. All errors must be resolved before the amendment can successfully be submitted.
- See Add Animal Handling Details for steps on working with animal handling details.
- Click OK.
