Overview
A Participating Site Application is used to track data with human subject research conducted at another institution where University of Michigan (U-M) is the IRB of record. New participating site applications are completed and submitted to the appropriate review committee(s). A new Participating Site Application may be started and saved for completion at a later time.
Navigation
The Participating Site Application can be accessed via:
- SITE link in the eResearch email notification OR
- Study Team Member's Home workspace in eResearch
Step-by-Step Process
- Click the Name of the Participating Site, OR click the SITE link in the eResearch email notification informing you that a Participating Site Application is awaiting your completion.
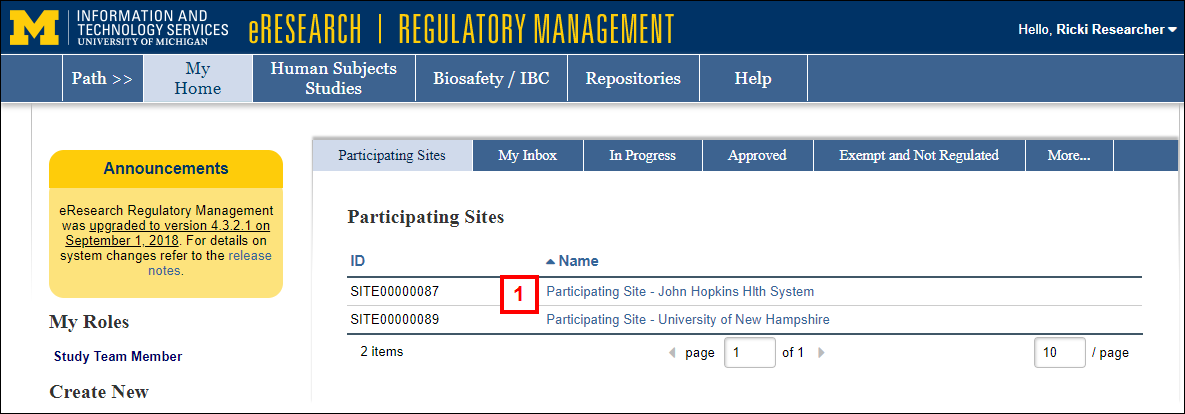
- Click Edit Project to open and complete the Participating Site Application.
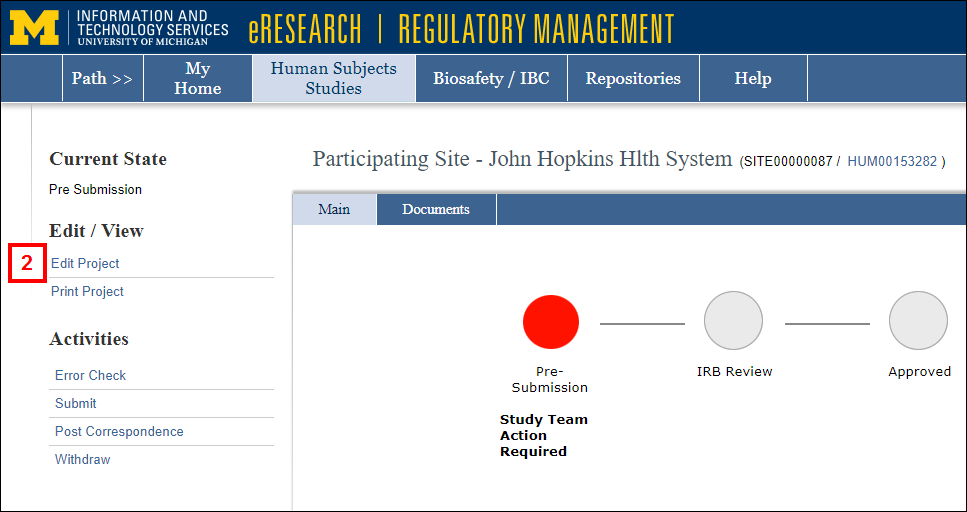
General Information Section
- Click add Add to add site team members.
Notes- Add site Co-Investigator(s), Lead Study Coordinator, one additional Study Coordinator, and one IRB Staff Member point of contact from your institution.
- Click edit Update to edit or delete Delete to remove an existing Site Team Member.
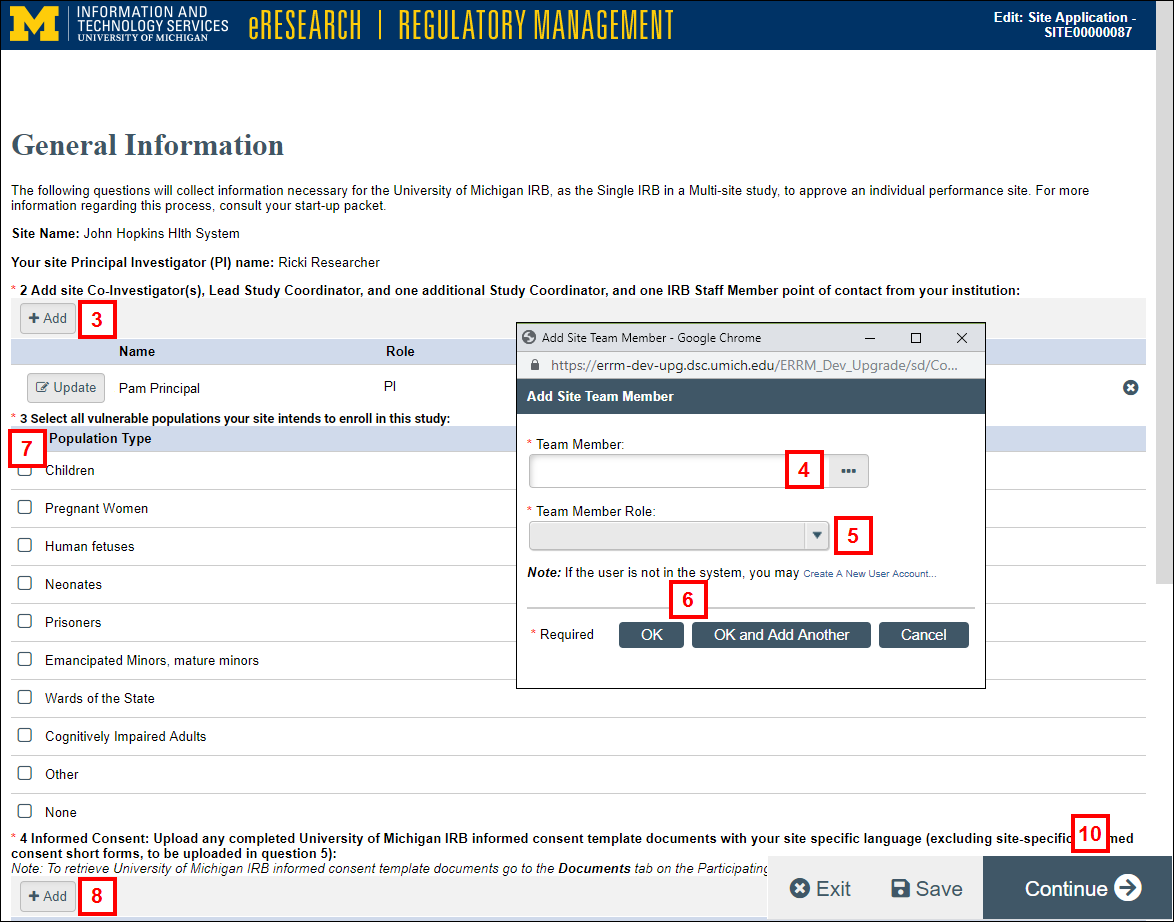
- Enter and select a Team Member.
Notes- Click
 to browse for a user by Last Name.
to browse for a user by Last Name. - If a user is not in the system, you can create a new user account.
- Click
- Select the Team Member Role.
- Click OK or OK and Add Another.
- Check the applicable vulnerable Population Type(s) your site intends to enroll in this study.
- Click Add and upload Informed Consent document(s).
Note Upload any completed University of Michigan IRB informed consent template documents with your site specific language (excluding site-specific informed consent short forms, to be uploaded in question 5).
To retrieve University of Michigan IRB informed consent template documents, go to the Documents tab on the Participating Site workspace and look for Study Wide Documents. - Complete all remaining questions. Additional questions may display, depending on the answers provided. Required fields are marked with an asterisk (*).
- Click Continue to save the current page and go to the next page.
Note An error message will display if any question's answers are incomplete.
IRB Section
important This section should be filled out or confirmed by your designated local IRB point of contact.
- Complete the remaining questions.

- Check the box to confirm The information provided in this application represents an accurate description of local context for the intended performance site.
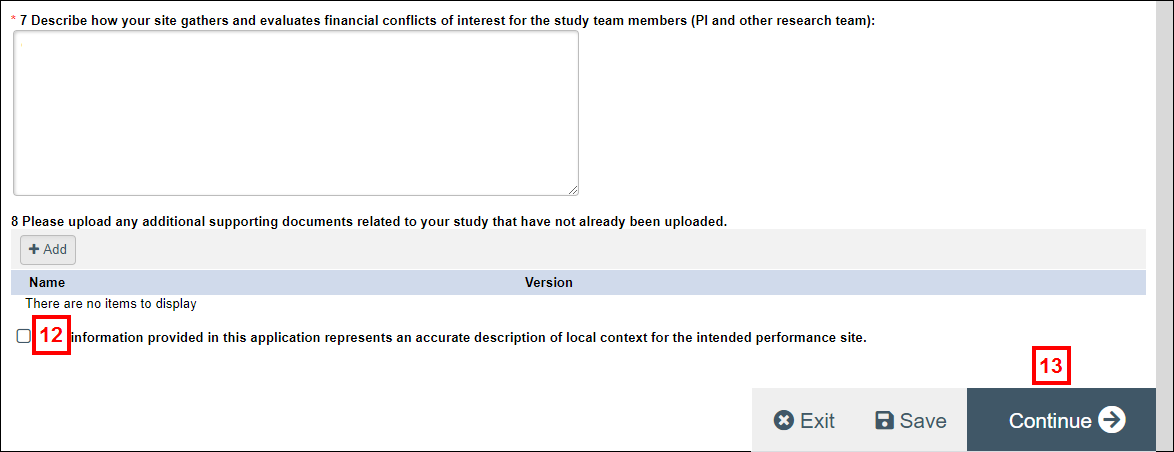
- Click Continue.
- Click Save & Exit.
Notes- Upon saving and exiting an application that is in progress, you are directed to the Participating Site workspace. Refer to Participating Site Workspace for more details.
- If the application is already completed, then the PI can submit it for review. Refer to Submit Participating Site Application for details.
Last Updated
Friday, February 19, 2021
