Overview
Only the Principal Investigator (PI) can submit a completed Participating Site Application. Before submitting, all required fields on the application must be complete.
Navigation
Role: Study Team Member (PI) > My Home > Participating Site workspace
Step-by-Step Process
After clicking the name of the Participating Site from your Home Workspace, complete the following steps:
- Click the Submit activity from the Participating Site workspace.
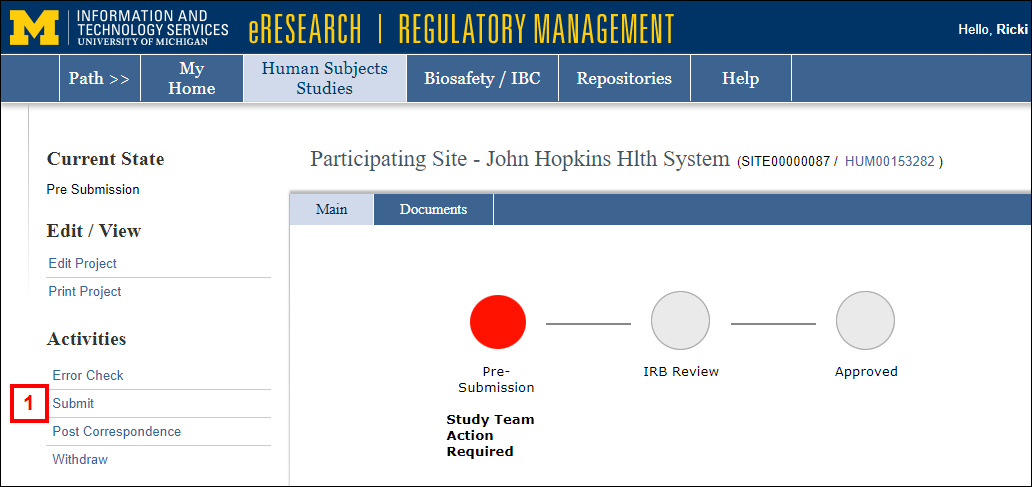
OR
Click Submit from the End of Application page of the Participating Site Application.

Note The system validates that all required fields are complete. You must correct all errors before proceeding. - Read the Investigator Assurances and check the box to confirm that you agree to abide by the assurance statement.
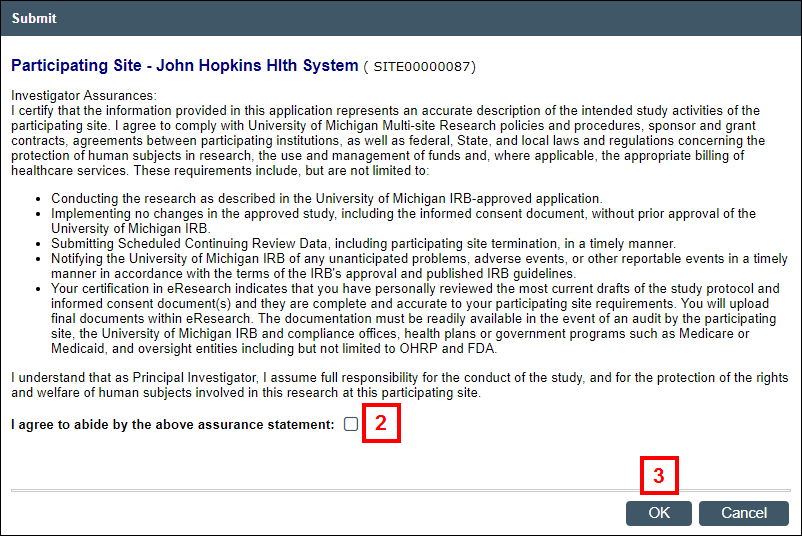
- Click OK to submit the Participating Site Application for review to the appropriate committee(s).
Note The state of the application changes to IRB Review.
Last Updated
Friday, February 19, 2021
