eResearch Proposal Management (eRPM) Version 5.3 - May 6, 2019
This document outlines the compliance changes occurring in the PAF Section 5 for Institutional Biosafety Committee (IBC) Approval, as well as updates to Export Controls/Classified Research.
In the Proposal Approval Form (PAF), changes are reflected in Section 5 - Research Activity, the PAF Summary, and in the Update Research Activity.
In Unfunded Agreements (UFAs), changes are reflected in the Material Transfer Agreements (MTAs) and Other, and in the UFA Summary.
PAF Section 5 - Research Activity Question Changes
Biosafety changes
Prior to May 6, 2019, questions 5.1 - 5.7 and applicable sub-questions were all required.
Old questions
- 5.1 Human research?
- 5.2 Use of human substances including cells/cell lines, blood products, body fluids, tissues, pathology materials, organs, body parts, cadavers?
- 5.3 Use or derivation of human induced pluripotent stem cells (iPSC) or human embryonic stem cells (hESC)? new 5.2
- 5.4 Use of vertebrate animals, including custom anitbody production? new 5.3
- 5.5 Use of recombinant or synthetic nucleic acid molecules (rDNA or SNA)? new 5.4.1
- 5.6 Use of biological agents or toxins on the Federal Select Agent and Toxin list? new 5.4.3
- 5.7 Use of biological agents infectious or hazardous to humans, animals, or plants? new 5.4.2
As of May 6, 2019, you can answer a new high-level question, then only answer applicable sub-questions. If any of the sub-questions are answered “Yes”, the additional detail questions display. You can link to an IBCA or indicate that an application is not yet submitted/pending.
New and revised biosafety questions
Four existing questions were revised (5.2, 5.5. 5.6. 5.7) and four questions were added, causing a renumbering of questions.
5.4 Does this project involve research in a U-M laboratory with biological materials?
- 5.4.1 Use of recombinant DNA (rDNA) or synthetic nucleic acid (SNA) molecules?
- 5.4.2 Use of infectious agents (i.e., bacteria, viruses, parasites, fungi, prions)?
- 5.4.3 Use of biological toxins (i.e., toxic substances produced by bacteria, fungi, protozoa, insects, animals, or plants)?
- 5.4.4 Use in a U-M research laboratory of human-derived substances (including cell/cell lines, blood products, body fluids, tissues, pathology materials, organs, body parts, cadavers)
- 5.4.5 Use of animal derived substances (i.e., cells, tissues, fluids from non-human primates, ruminants, swine, fowl, or any wild vertebrate animal)
- 5.4.6 Use of transgenic animals
- 5.4.7 Will any of the following be administered to vertebrate animals (rDNA, SNA, infectious agents, biological toxins, human‐derived substances (including cell/cell lines, blood products, body fluids, tissues, pathology, materials, organs, body parts, cadavers), animal‐derived substances (including cells, tissues, fluids from non‐human primates, ruminants, swine, fowl, or any wild vertebrate animal)?
- 5.4.8 Indicate the IBC Application(s) that cover the use of biological materials above in questions 5.4.1 - 5.4.7:
- Not yet submitted/Pending checkbox OR
- Add button
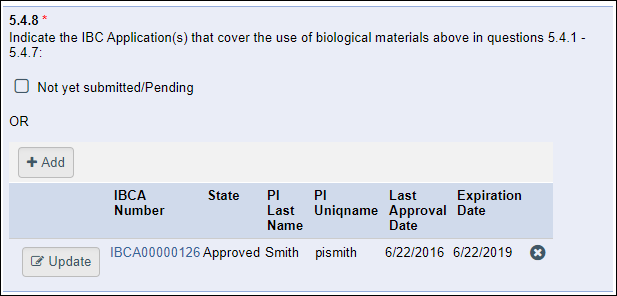
When completing the PAF, it is possible (and acceptable) to answer the high-level question “Yes” and “No” to all the sub-questions. If so, no IBCA is expected.
An approved IBCA will be required at the time of Award or Award Modification if the rDNA/SNA question is answered “Yes” for all Biosafety Levels (BSL). See Award and Award Modifications below.
Controlled Unclassified Information and Controlled Substance changes
The existing Export Controls, Classified Research and Enhanced IT Security question was split into two questions, and a new Controlled Substance question was added:
- 5.6 Does the research project involve possible export controls or delivery of a physical item, such as a product or material, including models and prototypes?
- 5.7 Are there any enhanced security requirements for this project (e.g., CUI, FISMA, or classified research)?
- If "Yes" or "Unsure", then a request will be sent to the U-M Security Officer for review when the PAF moves to the “Negotiation in Progress” or “Processing Award” states.
- 5.10 Use of a controlled substance (as defined by the federal Controlled Substances Act) or Propofol in a U-M research laboratory?
Update Research Activity
PI & Project Team personnel can use Update Research Activity on the PAF Workspace to complete the new required compliance questions and/or add associated HUMs, PROs, and/or IBCAs.
The Update Research Activity is available in states where the PAF is not editable for changes before ORSP creates an Award.
Award and Award Modifications
An IBCA will be required if the existing rDNA question is answered "Yes".
The Compliance Status meter will display red until IBC Approval is given, yellow if the IBC will be in the future, and green for all other biosafety conditions.

