Overview
A new Institutional Animal Care & Use Committee (IACUC) protocol application and submission form – commonly referred to as “V2” – is available in eRAM for select users. The form was evaluated, redesigned, and fine-tuned with the primary goal of minimizing the time and effort required of researchers and project teams to complete the form. Adoption of the new form will occur in stages throughout 2023.
Effective November 20, 2023, the pilot phase concluded, and Study Teams will no longer be able to create new or renewal protocols using the original version 1 “V1” application form.
Important Information about V1 and V2 protocols
- Approved V1 protocols will remain as is until they expire, per their 3 year approval.
- Any existing V1 protocol that is not renewed or transitioned to the new V2 application will expire and no longer be approved for animal use.
- As a V1 protocol approaches expiration, Study Teams will be prompted to renew their protocol using the new V2 template.
- Since the structure is very different, minimal information will automatically be copied over into the renewal application. Please proactively seek assistance from the Animal Care and Use Office (ACUO) Quality Assurance (QA) team when filling out a new V2 application form for the first time.
- Until November 20, 2023, only those pilot participants with the ACUO RCA Role will have the Create A New V2 Application button.
- As of November 20, 2023 (upon conclusion of the pilot phase), the Create New Application button will create V2 template protocols instead of V1 protocols, and you will no longer have the ability to copy V1 protocols.
What's Different
Key Changes that Researchers can expect
Reduced text entry and fewer number of procedures pages to complete
- Minimized the number of open text boxes throughout the application.
- Much of the form is now populated using drop-down lists (or pick-lists) and checkboxes, eliminating the need to enter duplicate and possibly conflicting, information across different protocol sections.
- Default text and pre-populated fields will include programmatic recommendations for common procedures (e.g., aseptic procedure; click “yes” to adhere to what is written, or modify as needed).
- Assurance statements included throughout with a “Yes” or “No” answer choice will require entering the rationale only if responding “No.”
- Increased flexibility to request research-specific plans and dosages (e.g., requesting a “maximum” number of injections instead of having to select specific values).
- Eliminated the Procedure Summary. Instead, the “Sequential Description of Animal Activities” outlines the procedures to be conducted, and details of procedures are entered in the subsequent procedure pages.
Leveraging data entered for other users
- Names of substances in eRAM now match the names used in Institutional Biosafety Committee Application (IBCA).
- Using the entry of Agents and Substances in specific procedures to create an agent/substance summary table, which lists all agents and substances by procedure (for quick viewing and verification).
Redesigned procedure details
- Procedures are now pages (vs. popups/note pages).
- An “Other” procedure can be utilized if a procedure does not precisely fit within the predefined list of Procedures.
- If multiple species are included in a single protocol (PRO), then shared procedures are listed only once in the Navigation menu.
- The Procedure Summary Page for V1 has been replaced with the new V2 "Sequential Description of Activities and Estimation of Animal Numbers" page. It is very different in format and structure. Please proactively seek assistance from the Animal Care and Use Office (ACUO) Quality Assurance (QA) team when filling out this page for the first time.
- See New IACUC Protocol Application Form for how to contact the QA team.
Version 2 Application
The V2 protocol application has five high-level sections, each with applicable sub-sections:
|
 |
What's the Same
- Protocol Applications have a naming convention of Animal Protocol - PRO00001234.
- You can navigate through the V2 application in the same way you would as navigating the V1 application.
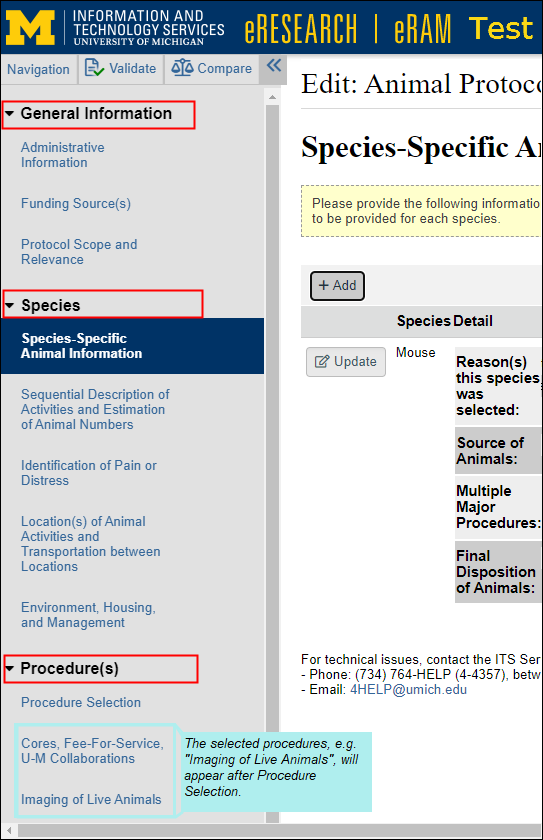
- A Navigation menu displays a list of all the pages in an application.
- The entire left navigator can be toggled on or off, (closed << or opened >> ) with the double-arrow icon.
- The page that you are currently viewing is highlighted, in bold.
- Buttons to exit Exit, save Save, and Continue right arrow (or Finish) "float" at the bottom of the page.
Tip Remember to scroll all the way down to answer additional questions.
- A Navigation menu displays a list of all the pages in an application.

- check Validate runs project validation and shows Error/Warning Messages. Pages/questions with green checkmarks checkmark indicate verification/completion; red circles minue indicate incomple or warnings.
- See the Validate job aid for more details.
- compare Compare to view changes between application versions and pages, once more than one version has been submitted.
- See the Compare job aid for more details.
- left Go to Navigation shifts the focus from the smartform to the navigation menu, i.e., it opens the navigation menu if it was collapsed.
- comment Reviewer Notes and comment Reviewer notes legend are located at the top-right corner of the application.
Note Reviewer notes only appear on animal protocols after reviewer notes/questions have been added. - When entering information for some questions that have Add, Update, or (browse)... buttons, additional fields to complete will slide in from the right side of the application. For example, when clicking Add to input details, another screen overlaps (similar to a pop-up box).
- Notes The dialog box can be minimize minimized or close closed to return to the main smartform, but you must click OK to save your selection.
- The main smartform controls remain locked for editing until you close the slide-in dialog box.
- If you have minimized a dialog box and returned focus to the main smartform, then options to close Close and restore Restore dialog boxes display in a bar on the right-side of the application.

- Dialog Box Example
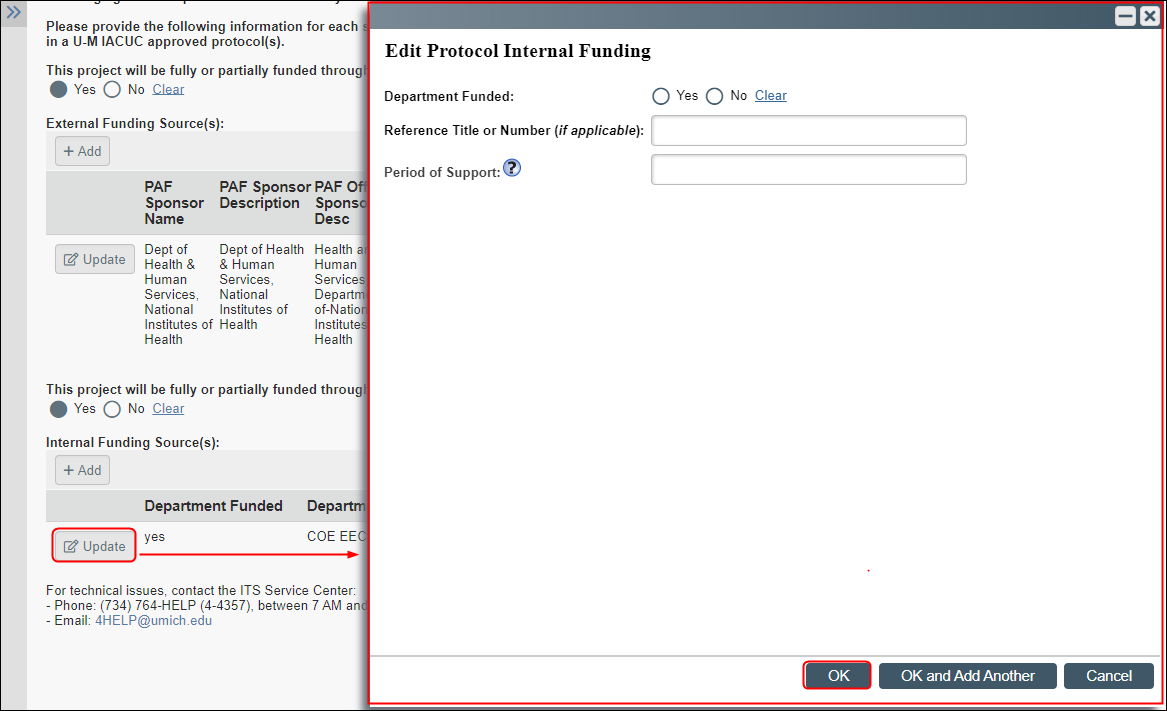
- Notes The dialog box can be minimize minimized or close closed to return to the main smartform, but you must click OK to save your selection.
- All questions that display are required. Depending on the response to the question, subsequent questions and related sections may display. These questions and sections provide the ACUO and other associated compliance offices with the detail about the research and precautions needed.
- Tip Click Continue to advance to the next required page in the application.
- Use Filter by to narrow the list of choices and results when searching.
- Click +Add Filter to filter by multiple search criteria.
- use % as a wildcard
- Click a column heading to sort by that field in descending order. Click again to sort by ascending order.
- Home workspaces and Protocol workspaces remain unchanged.
- Protocols can be submitted from the Protocol workspace using the "Submit Application" activity.
- The IACUC review process remains unchanged.
Additional Resources
For a list of step-by-step instructions, refer to the Version 2 Protocol Reference Materials.
