Overview
Only the PI can submit a completed study application. Before submitting, all required fields on the application must be complete, all applicable members of the study team must accept their role, and the application must be moved to Ready-to-Submit. It is recommended that you run the Error Check and Application Checklist activities before submitting the application. For more information, see Preparing an Application for Submission.
Navigation
Role: PI > Home Workspace > Study Workspace
Step-by-Step Process
After clicking the name of the submission from the Ready to Submit section of your Inbox, complete the following steps:
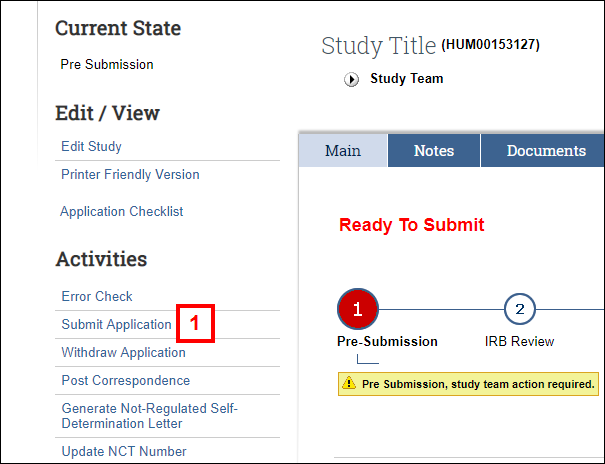
- Click the Submit Application activity.
Notes- You can also click Submit Application from section 45 End of Application of the Study Application.
- After clicking Submit Application, the system will automatically check for errors. You must address all errors before proceeding.
- (Optional) Click Upload Revision to update any documents listed in the Credentials section, or click Add to upload any new documents.
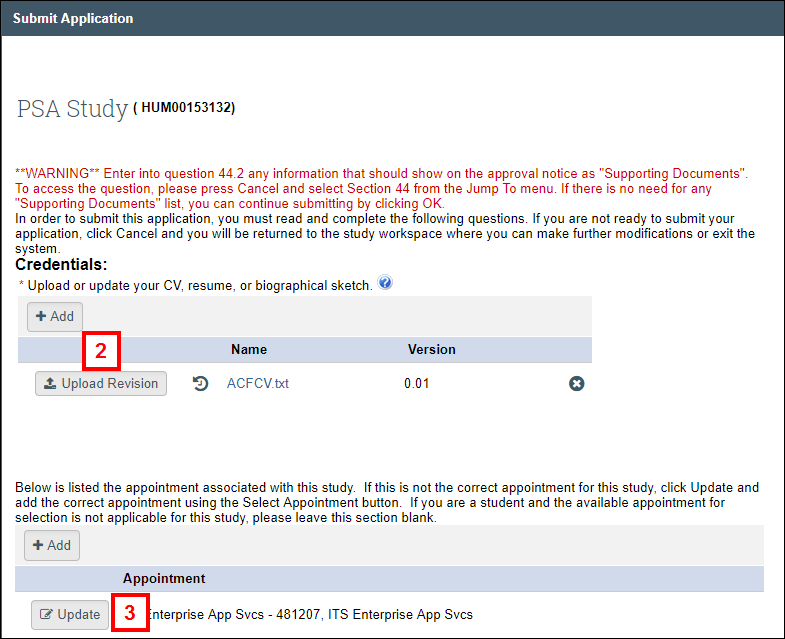
- (Optional) Click Update to update the appointment associated with the study.
Note If the appointment selection did not occur automatically, click Select Appointment. - Note your Current Disclosure Status in M-Inform.
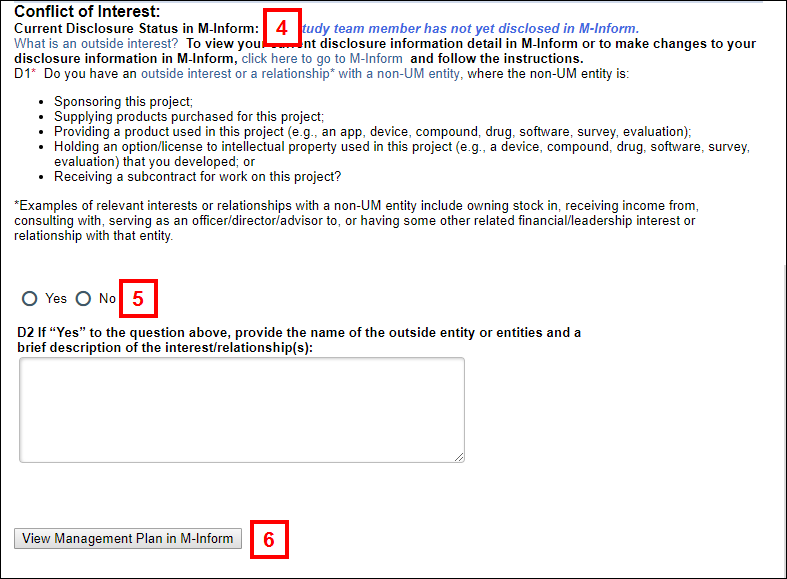
- Read COI question D1 and respond by clicking the applicable Yes/No radio button. If your answer to D1 is “Yes,” complete question D2.
Notes Depending on a combination of your current disclosure status and your response to the COI question, you may be required to update your disclosure in M-Inform before submitting.- In ALL cases where you answer “no,” you can submit the application.
- If you answer “yes” and have not disclosed, you have to disclose in M-Inform before you can submit.
- If you answer “yes” and have disclosed but have no outside interests, you have to update your disclosure in M-Inform before you can submit.
- If you answer “yes” and have disclosed and have outside interests, you can submit the application but will see a pop-up warning that says update your disclosure information, if necessary.
- (Optional) Click View Management Plan in M-Inform to verify that one exists, or in order to update your current disclosure in M-Inform.
- Check the box to indicate that you agree to abide by the Investigator Assurance statement.
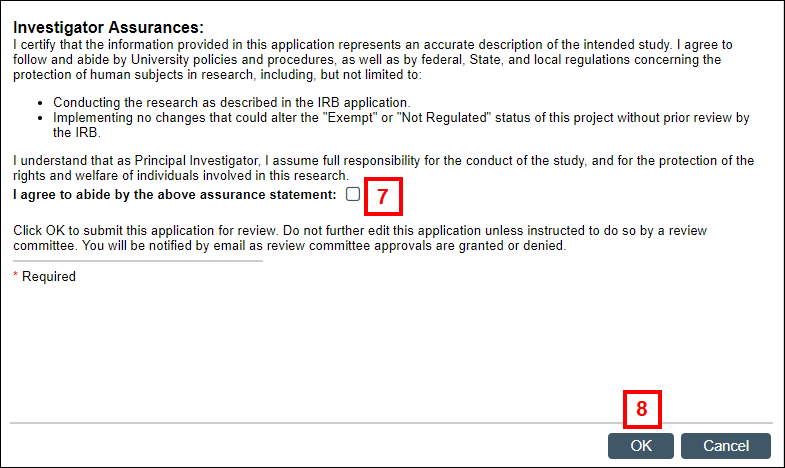
- Click OK to submit the application for review to the appropriate committee(s).
Last Updated
Monday, October 21, 2019
