Overview
Before the final agreement can be executed, the Contact PI must “sign” the UFA and a corresponding Conflict of Interest Statement before routing to ORSP. This process is completed using the Sign UFA activity in the UFA workspace.
Important Information
“Signing” the UFA does not route it for review. Before the UFA can be routed for review, the Project Team Route UFA activity must be executed (by the PI or Project Team). Refer to Unfunded Agreements for more information.
Navigation
Role: PI & Project Team > My Home
Step-by-Step Process
- Click the UFAs tab.
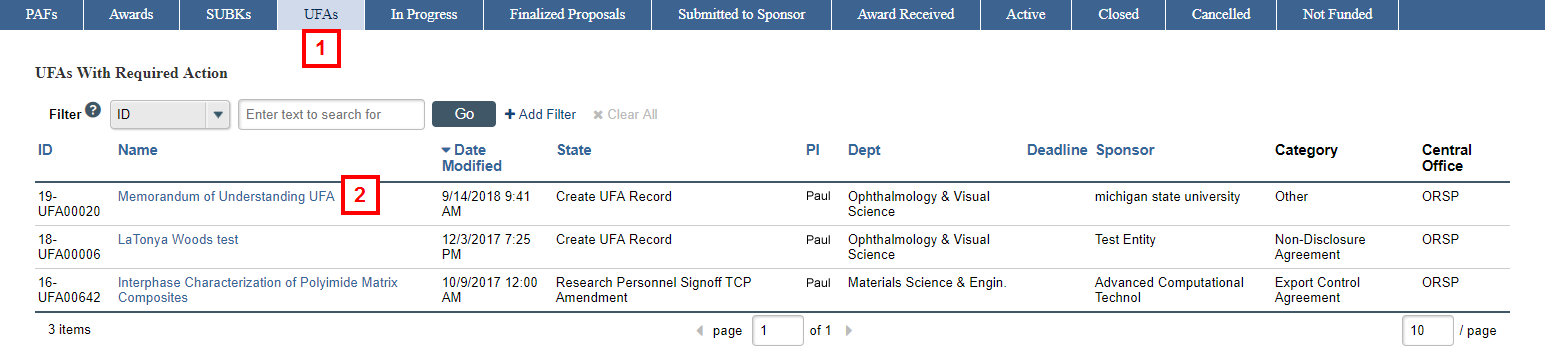
- Click the Name of the agreement in the UFAs Not Yet Signed by PI list.
- Click the Sign UFA activity on the UFA workspace.
exclamation A yellow highlighted message alerts you that the UFA must be signed by the Contact PI before it can be activated.
- Select the applicable radio button in response to the Conflict of Interest question.
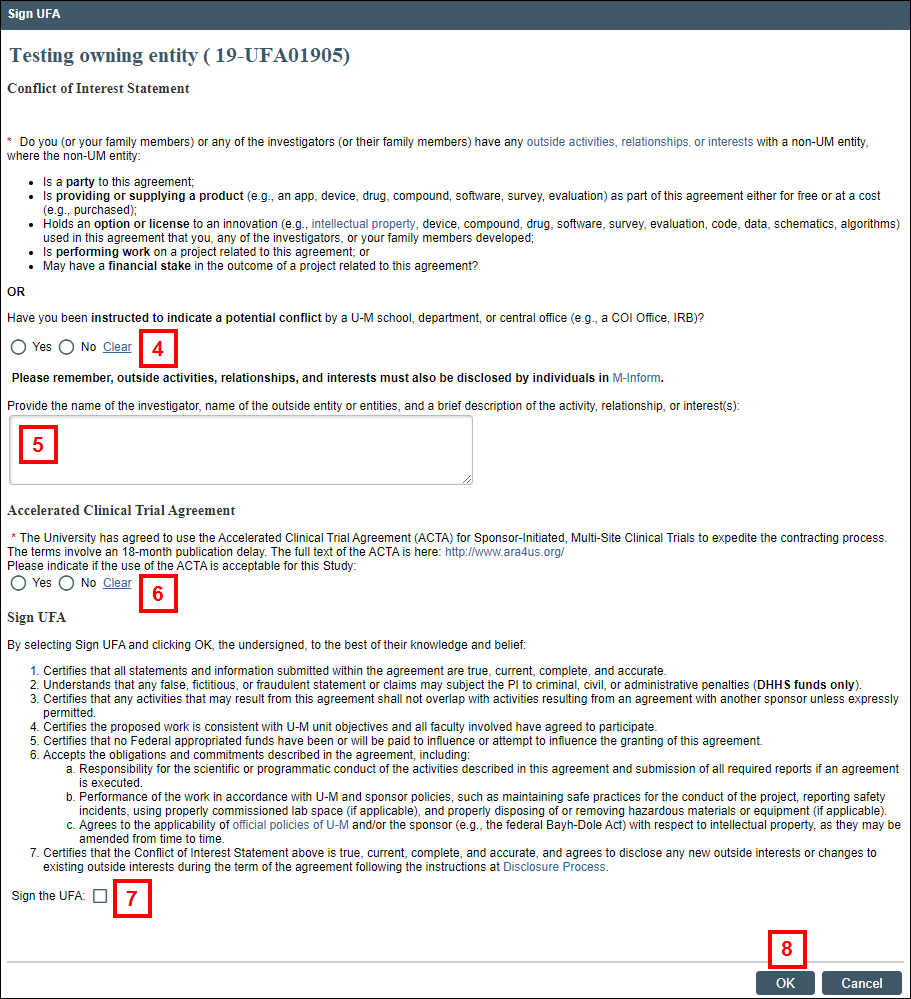
- Enter the name of the investigator, name of the outside entity or entities, and a brief description of the interest/relationship(s), if applicable
Note An answer is required if you answered Yes to the Conflict of Interest question. - If applicable, click the radio button in response to the Accelerated Clinical Trial Agreement question. (This question only displays for Clinical Trials which are Sponsored Trials – Multisite.)
- Check the Sign the UFA box to electronically sign the UFA.
- Click OK.
Note The yellow highlighted message no longer appears in the UFA workspace.
Last Updated
Friday, August 23, 2024
