Overview
Once a Participating Site Application has been approved, an Amendment must be created to document changes (e.g., changes to team members, subject populations, etc.).
After submission, the Amendment application is locked and no further changes can be made unless requested by a reviewer. Only one Amendment can be in process at a time.
Once approved, the Amendment is accessed through the original application under the Amendments tab.
Important Any Study Team Member listed on the approved Participating Site Application can initiate an Amendment, but only the U-M Principal Investigator can submit it for review.
Navigation
Role: PI/Study Team Member > My Home
Step-by-Step Process
Create an Amendment
- Under the Participating Sites tab or the Approved tab, click the Name of the Participating Site Application you want to amend.
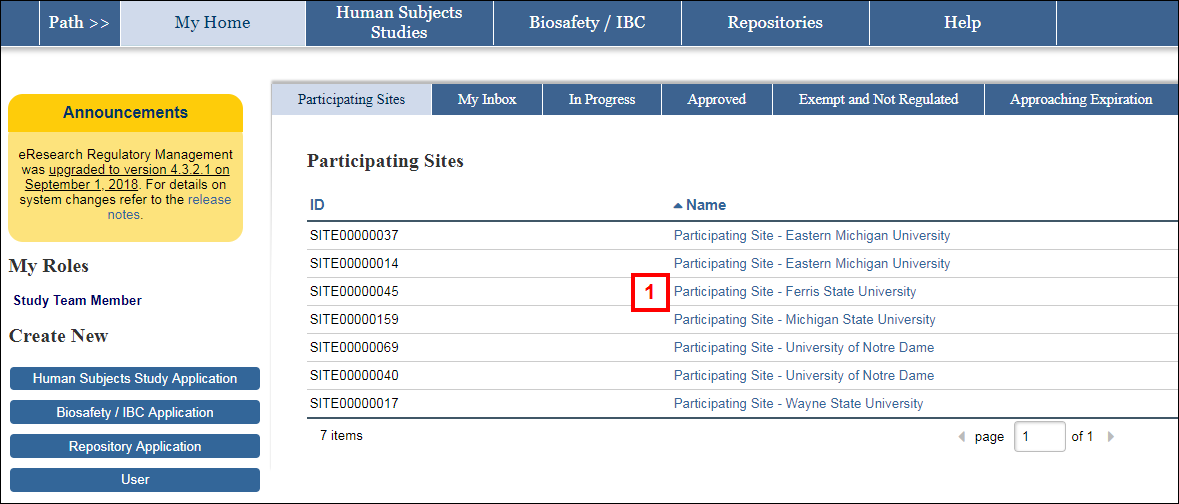
- Click the Amendment button.
Note If the Amendment button does not display, another amendment is already in progress.

- Enter a Participating Site Amendment Title.
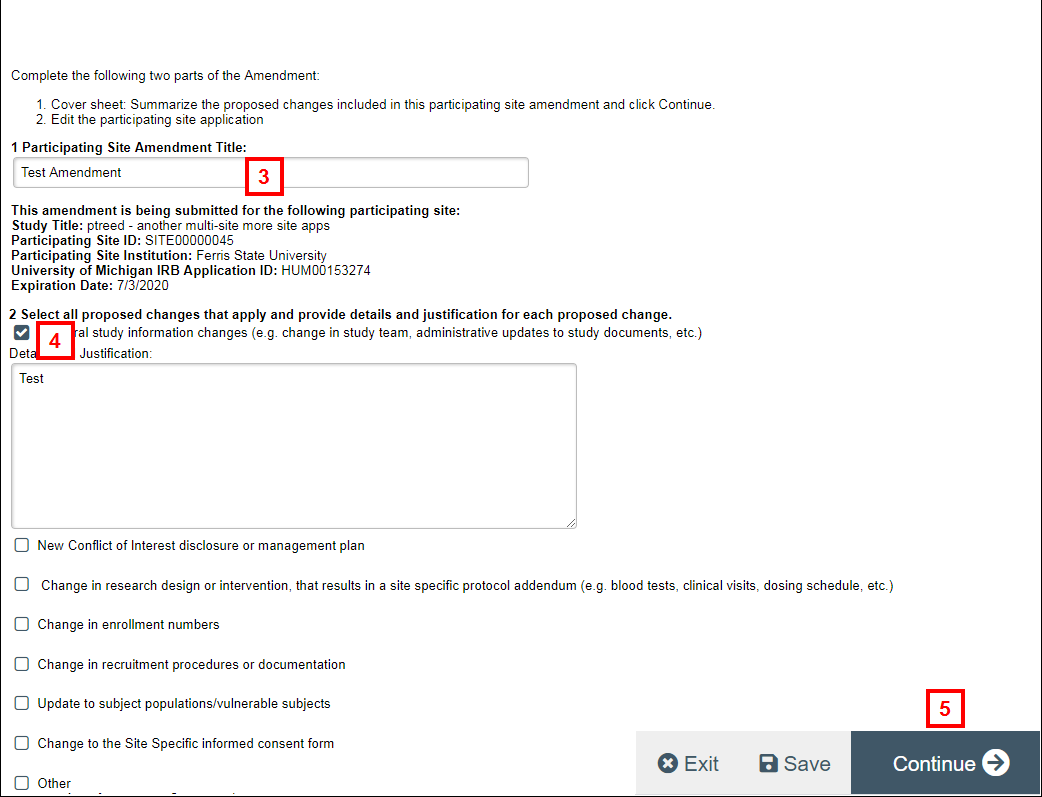
- Check all proposed changes that apply and enter Details and Justification for each proposed change.
- Complete remaining fields, then click Continue.
Note A clone of the application opens with an assigned amendment (AME) ID number. Upon approval, these changes are copied into the original application. - Revise any fields that need to be changed.
Notes- If adding a new study team member who is not in the system, you can create a new user account.
- The IRB Section should be filled out or confirmed by your designated local IRB point of contact.
- When finished, click Continue until you get to the end of the form, or click Forms Menu to go to the End of Application page.
- From here, you can click Save & Exit to go to the Amendment workspace. OR
Click Submit to open the submit activity, then go to step 10.
Submit an Amendment
Only the Principal Investigator can submit an application’s amendment.
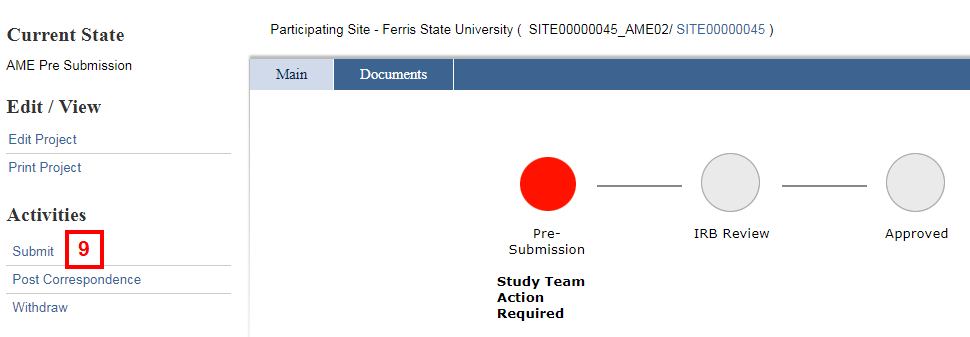
- Click the Submit activity from the Amendment workspace.
Notes- The status of the Amendment is AME Pre-Submission.
- The system validates that all required fields are complete. Any errors must be addressed before the Amendment can be submitted.
- Read the Investigator Assurance statement, then check the box to indicate that you agree.
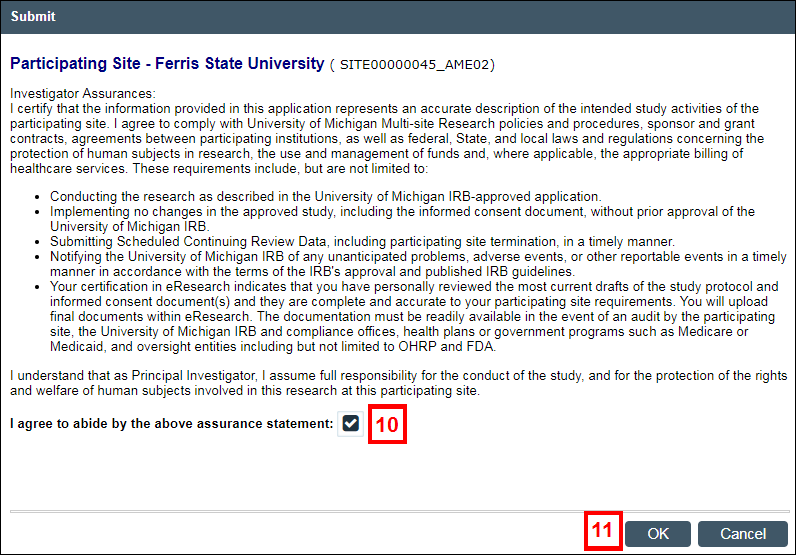
- Click OK to submit the Amendment for review by the appropriate committee(s).
Note The status of the Amendment changes to IRB Review.
Last Updated
Friday, February 19, 2021
