Overview
Once a study application has been approved, an amendment must be created to document changes to the study (e.g., changes to study team, sponsors, subject populations, etc.). An amendment can be initiated by any Study Team Member listed on the approved application, but it can only be submitted for review by the UM Principal Investigator. After submission, the amendment application is locked and no further changes can be made unless requested by a reviewer. Only one amendment can be in process at a time for a study.
Important Information
- In addition to any changes related to the amendment, you must also verify that the required HR appointment information is provided for each study team member listed on the study before submitting the amendment.
- If the required appointment information is not provided, you will receive an error and will not be able to submit the amendment. For more information regarding study team member appointments, see Adding a Study Team Member.
Navigation
Role: PI/Study Team Member > Home Workspace
Step-by-Step Process
- Click the Approved tab.
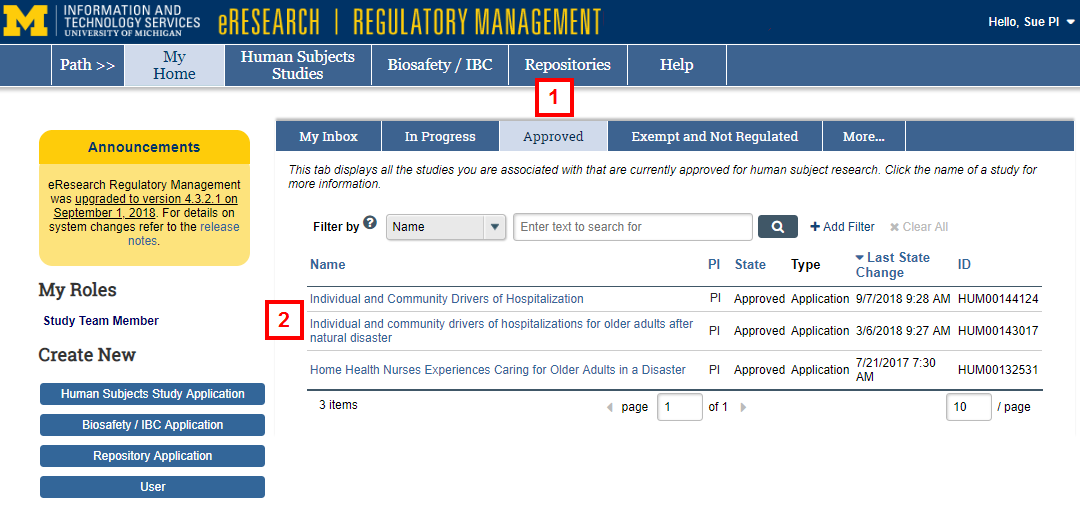
- Click the Name of the study.
- Click Amendment.
Notes- If the amendment is in response to an adverse event (AE) or an other reportable information and occurrence (ORIO) that has not yet been submitted for review, click Adverse Event/ORIO instead.
- AE/ORIOs must be submitted prior to the initiation of the amendment in order for them to automatically display in the amendment cover sheet. This allows the reviewer to consider the amendment in the context of the AE/ORIO report.

- Click Continue after reading the amendment Instructions.

- If applicable, enter an Amendment Title. Leave the original study number in the amendment title unless directed by the IRB to follow a specific naming convention.
Note Once approved, the amendment is accessed through the original study.
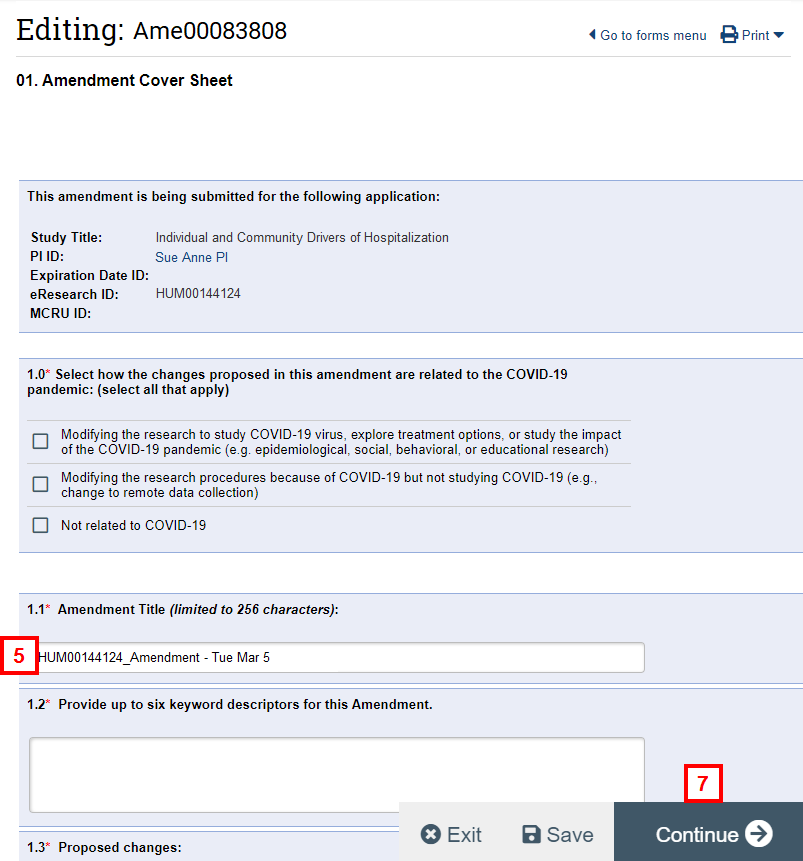
- Complete the remaining fields on the form.
- Click Continue.
- Verify the boxes are checked next to the names of the study team members who should receive email notification(s).
Note This page informs you that the system is currently creating a copy of the approved application. The system notifies the selected study team members via email when the amendment copy process is complete.
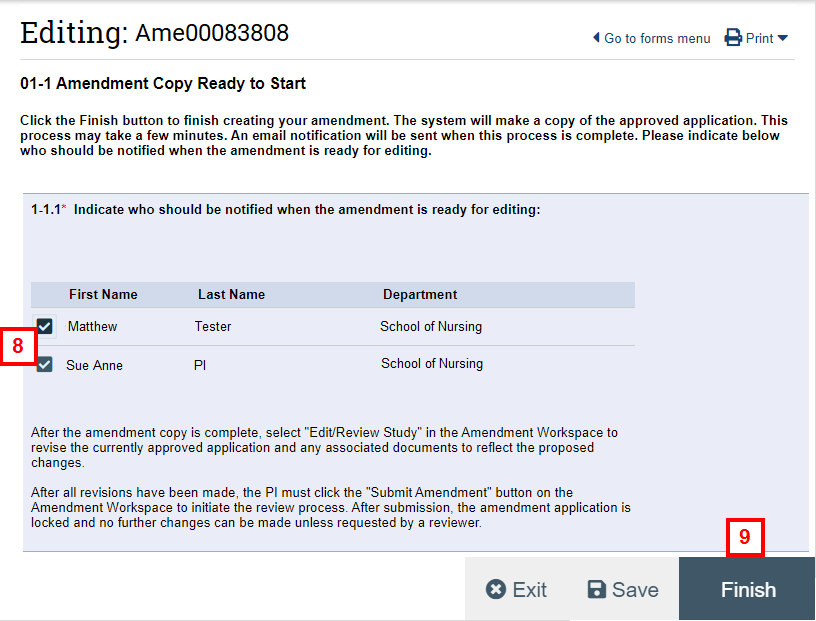
- Click Finish.
Note The system indicates that it is creating the amendment. - After the amendment is created, navigate to the Amendment Workspace.
- Click Edit Study to amend the study.
Note If adding study team members in the amendment, any new Co-Investigators and Faculty Advisors will need to accept their role prior to submission.
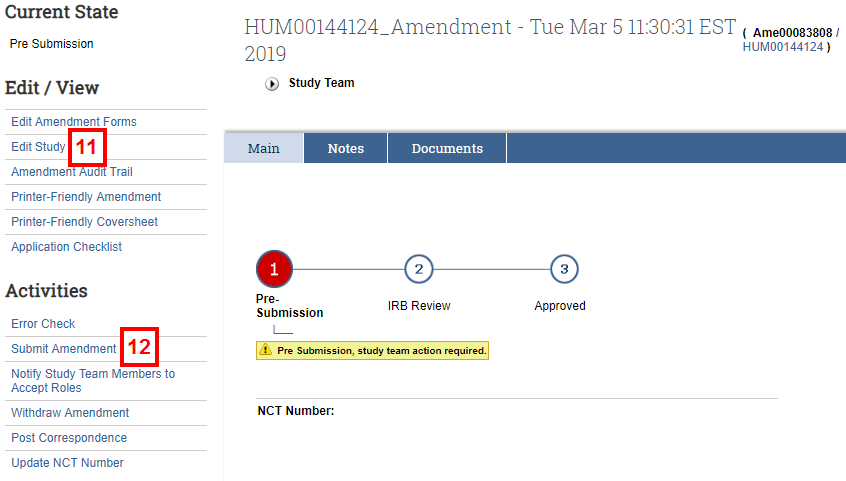
- After making all changes to the amended application, click the Submit Amendment activity.
Note Only the PI can submit an amendment. - If applicable, click Upload Revision to update any documents listed in the Credentials section, or click Add to upload any new documents to the section.
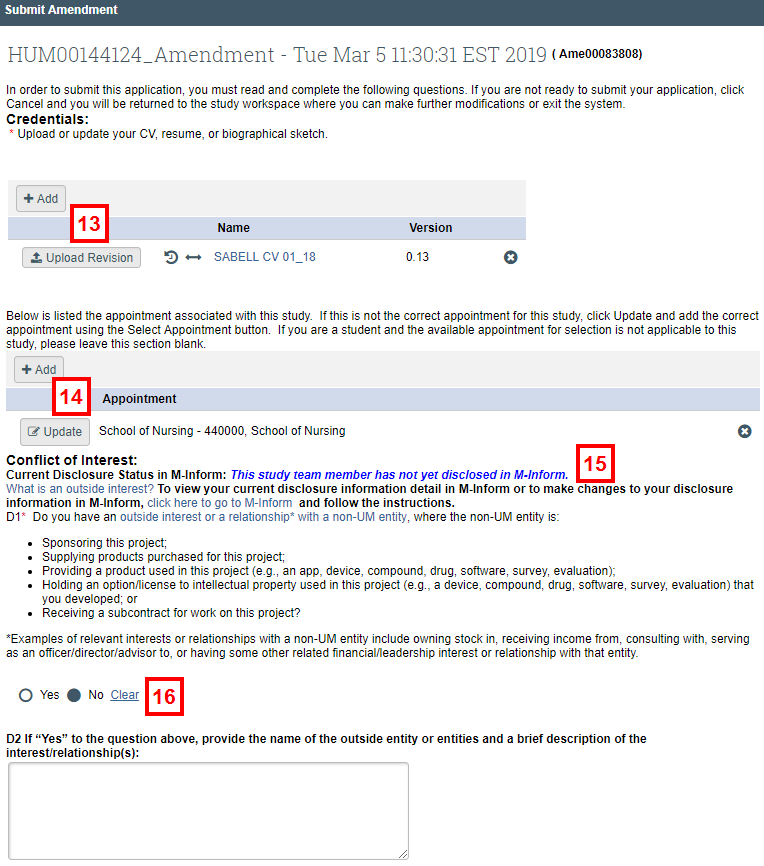
- If applicable, click Update to update the appointment associated with the study. This option only displays if an appointment was previously selected.
- Note your Current Disclosure Status in M-Inform.
- Read COI question D1 and respond by clicking the applicable Yes or No radio button. If your answer to D1 is Yes, complete question D2.
Notes- Depending on a combination of your current disclosure status and your response to the COI question, you may be required to update your disclosure in M-Inform before submitting.
- Disclosure Status / COI Question Response Scenarios
- In ALL cases where you answer No, you can submit the amendment.
- If you answer Yes and have not disclosed, you will have to disclose in M-Inform before you can submit.
- If you answer Yes and have disclosed but have no outside interests, you will have to update your disclosure in M-Inform before you can submit.
- If you answer Yes and have disclosed and have outside interests, you will be able to submit the amendment but will see a pop-up warning that says if necessary, update your disclosure information.
- If applicable, click View Management Plan in M-Inform to verify that one exists, or in order to update your current disclosure in M-Inform.

- Check the box to indicate that you agree to abide by the Investigator Assurance statement.
- Click OK to submit the amendment for review by the appropriate committee(s).
Last Updated
Monday, February 15, 2021
