Overview
A Continuing Report (CR) is used to renew the approval of a participating site application. Study teams receive an email reminder that their site application is due for a scheduled continuing review 30, 60, and 90 days prior to the study expiration date.
A CR can be created by personnel with edit rights on the approved participating site application, but only the Principal Investigator can submit it for review. Only one Termination Report or Continuing Report for a site application can be in progress at a time.
Navigation
Role: PI/Study Team Member > My Home
Step-by-Step Process
- Click the Participating Sites or Approved tab on your Home page.
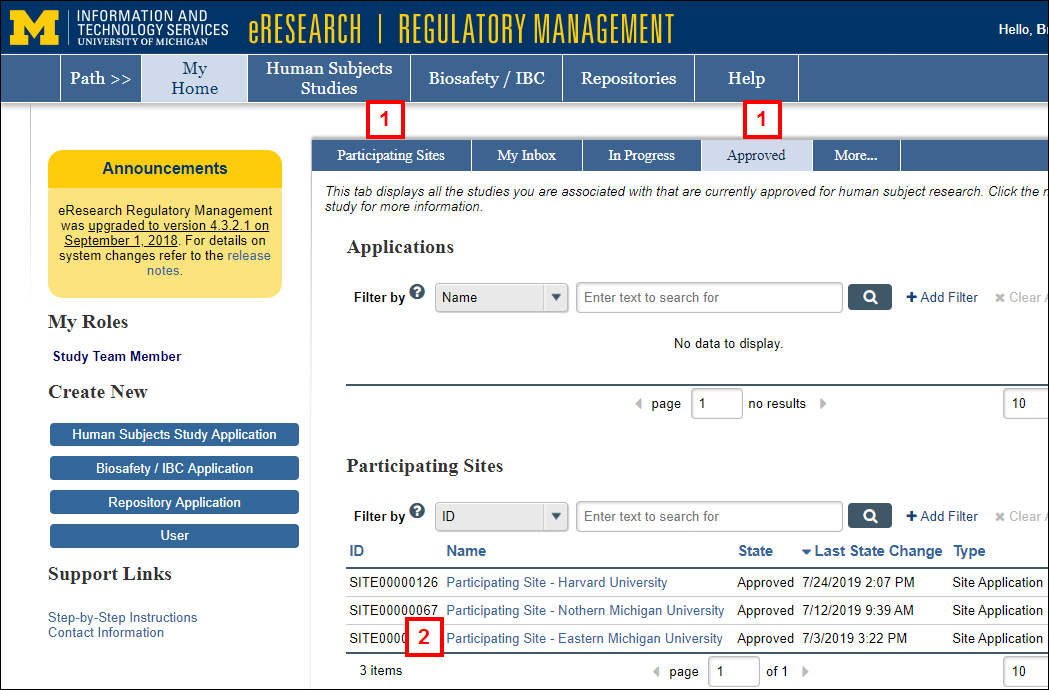
- Click the Name of the Participating Site Application.
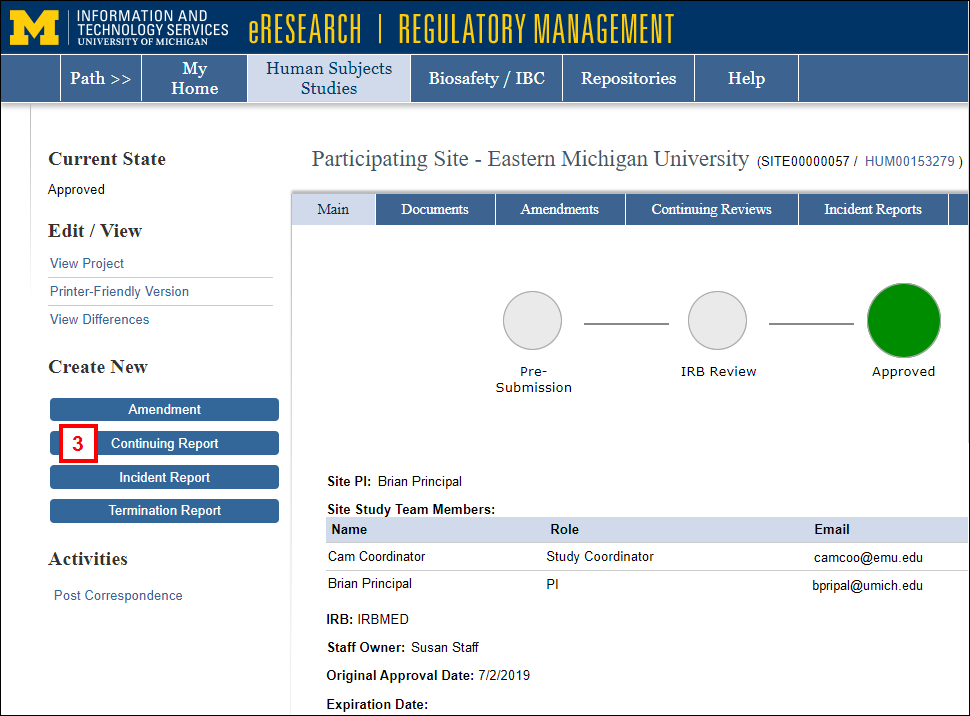
The State of the application must be Approved. - Click the Continuing Report button under Create New.
- Complete the required questions on each page of the Continuing Report.
Note Depending on the answers selected, additional questions may appear.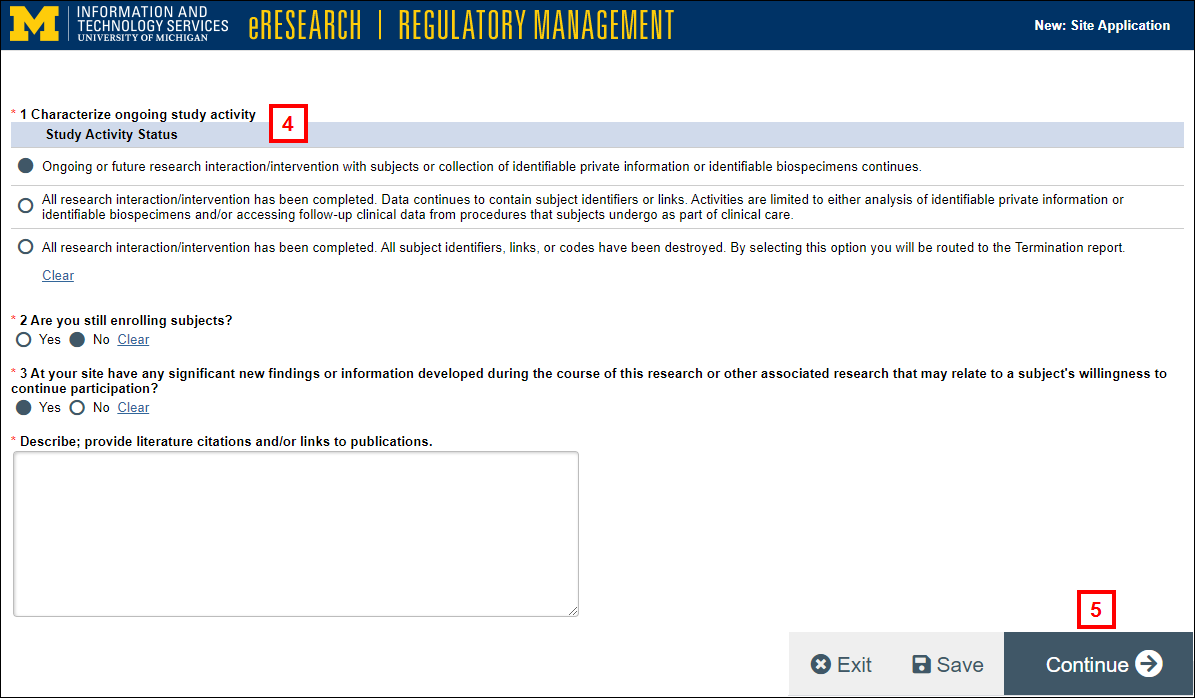
- Click Continue and complete the remaining questions and pages.
- Click Submit.
Notes- If you are not ready to submit, click Save & Exit. You will be returned to the Participating Site CR workspace. From there, click the Submit activity to submit at a later time.
- The system validates that all required fields are complete. Any errors must be resolved before the CR can be submitted.

- Read the Investigator Assurances, then check the box to indicate that you agree.
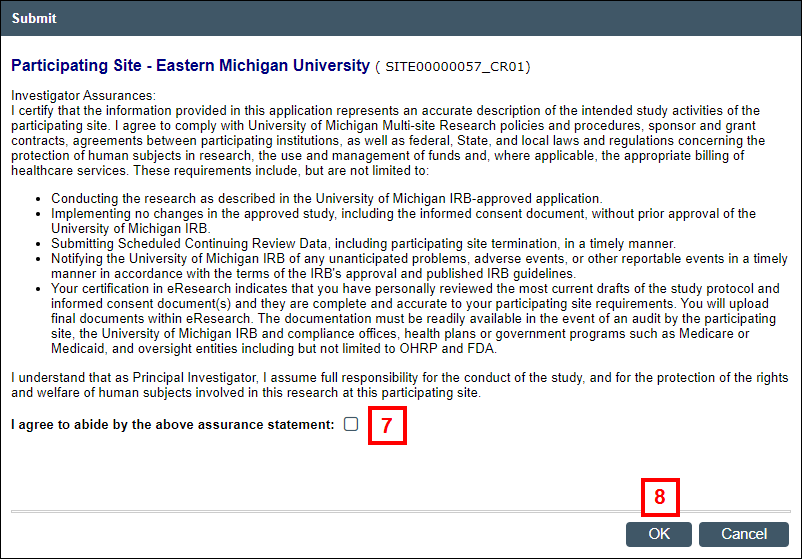
- Click OK.
Notes
- The State of the CR changes from SCR Pre Submission to IRB Review.
- Track the progress of a CR on the In Progress tab of your Home page.
- Once the Continuing Report is approved, go to the Continuing Reviews tab on the Participating Site workspace to view the CR.
Last Updated
Friday, February 19, 2021
