Overview
An Incident Report is submitted when a significant incident occurs that requires acknowledgment by the IRB. Adverse Events (AE) include any experience or abnormal finding that is harmful or increases the risk of harm to research participants. Other Reportable Information or Occurrence (ORIO) includes audits, other reports, protocol deviations, protocol violations, facility/data accidents, and complaints (which includes lapses in approval).
Note The basic steps for initiating an Incident Report are the same regardless of the type of report; however, the report form that opens and the information you are asked to provide will depend on the type of report selected (Adverse Events or Other Reportable Information or Occurrence).
Important An Incident Report (IR) can be created by personnel with edit rights listed on the approved participating site application, but only the Principal Investigator can submit it for review. After submission, the IR is locked and no further changes can be made unless requested by a reviewer.
The following procedure is a general guide for reporting an Adverse Events Report type.
Navigation
Role: PI/Study Team Member > My Home
Step-by-Step Process
Create an Incident Report (Adverse Events Report)
- Click the Participating Sites or Approved tab on your Home page.
Note An Incident Report can also be submitted for a Closed application.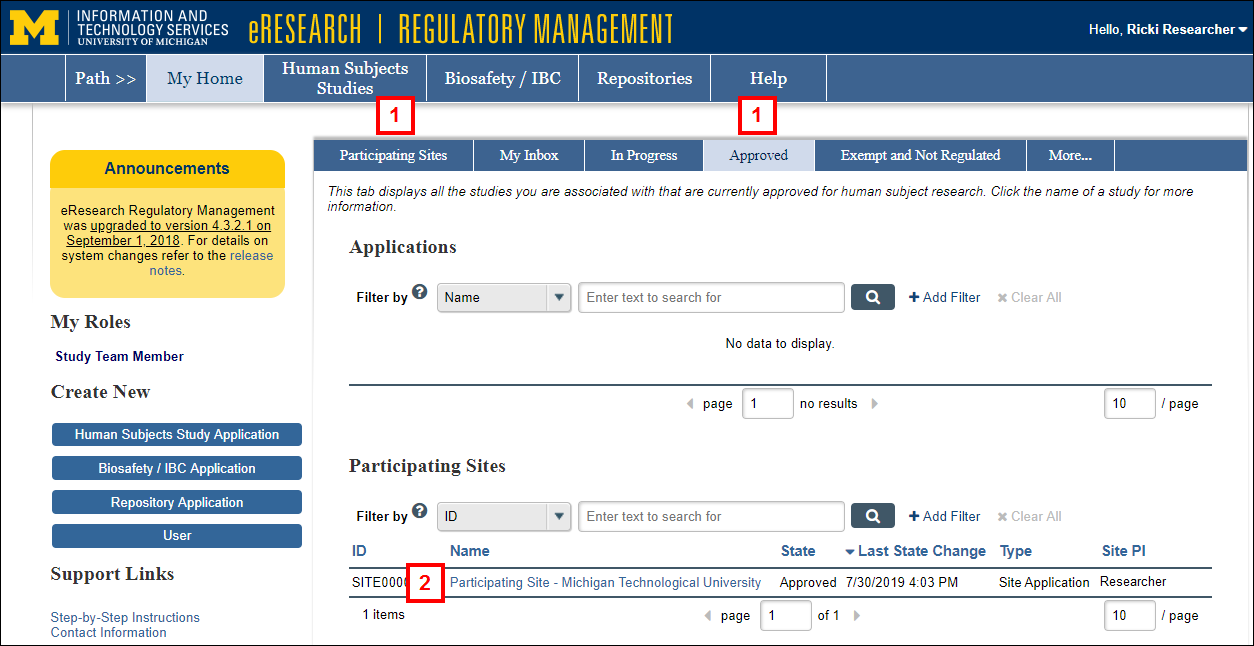
- Click the Name of the Participating Site Application.
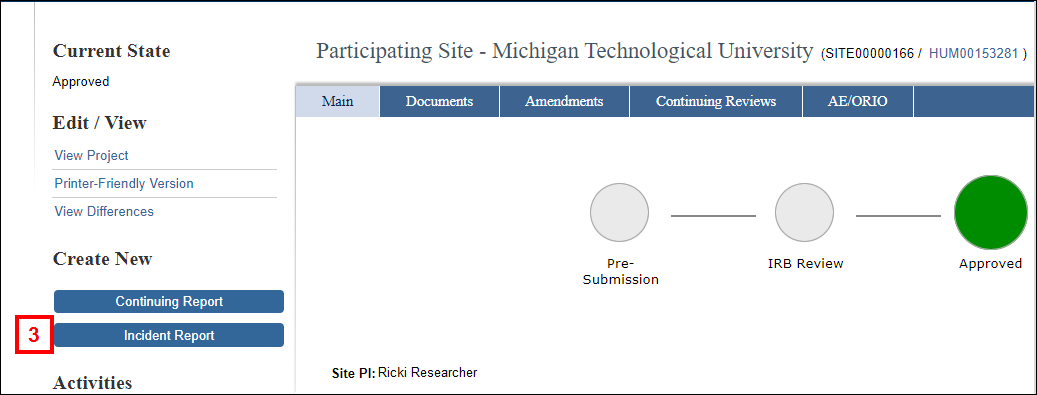
- Click the Incident Report button under Create New.
- Answer the required questions.
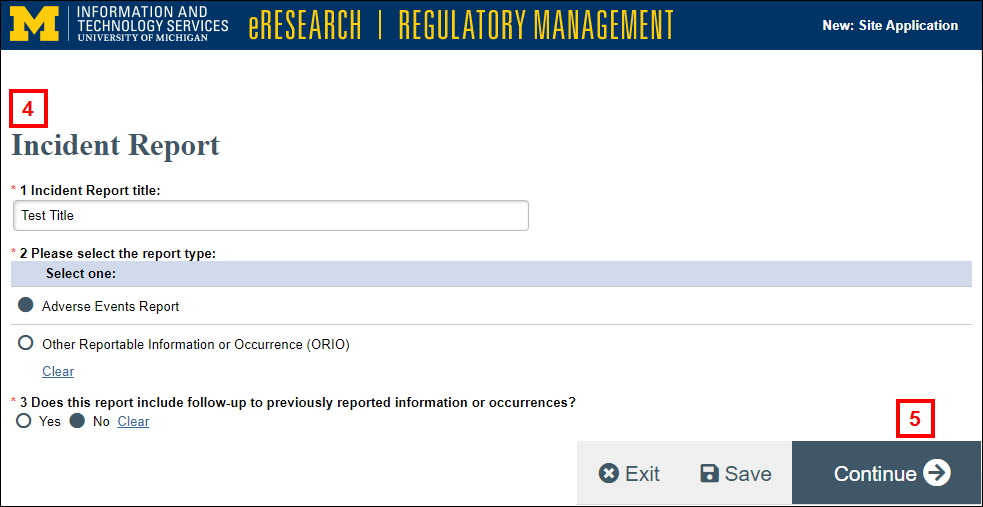
- Click Continue. Depending on the type of incident you are reporting, the applicable report form will open.
- Complete the required information on each page, and click Add to enter additional details.
Note Depending on the answers selected, additional questions may appear.
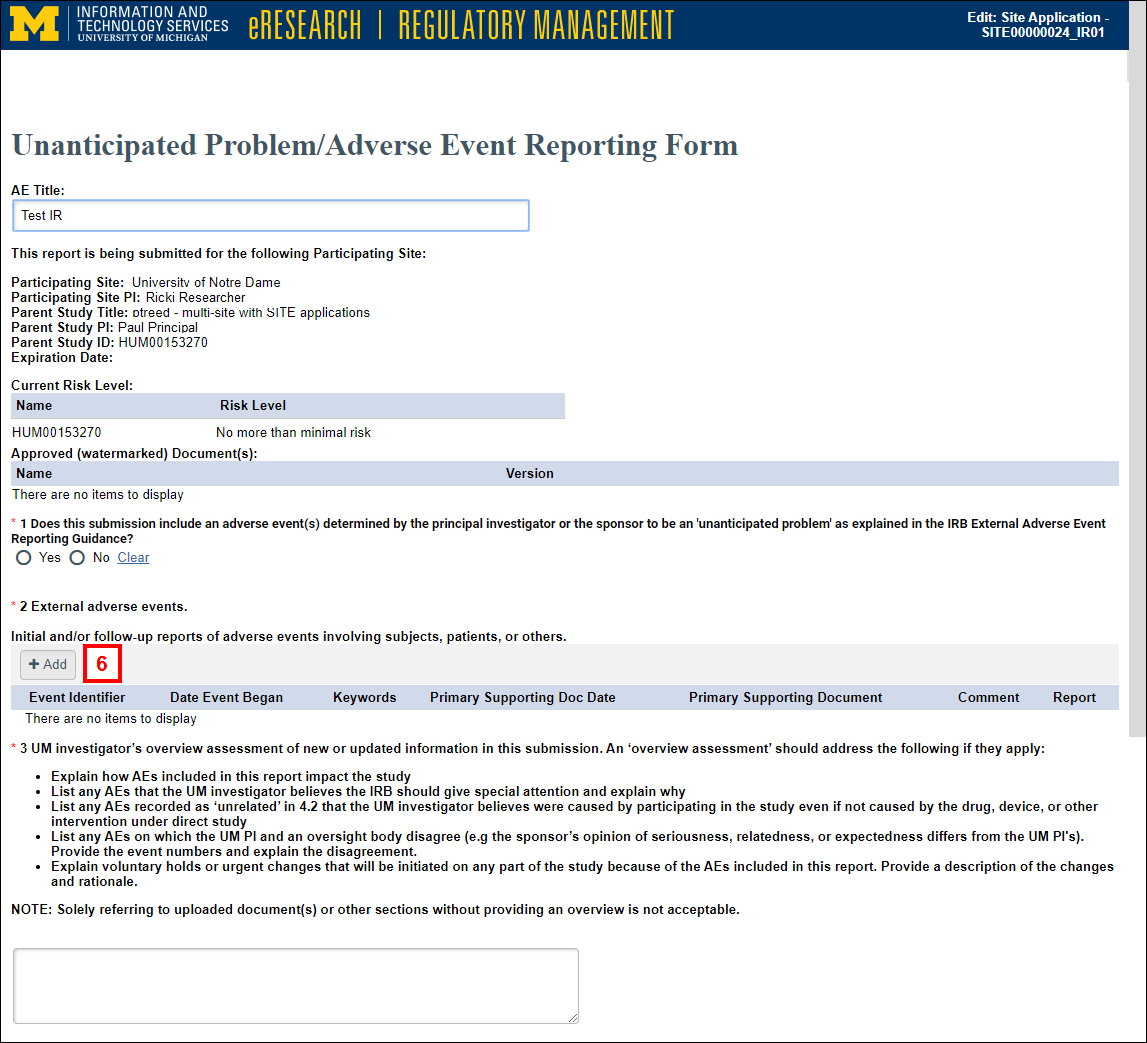
- Enter Adverse Event Details, then click OK or OK and Add Another at the bottom of the window.
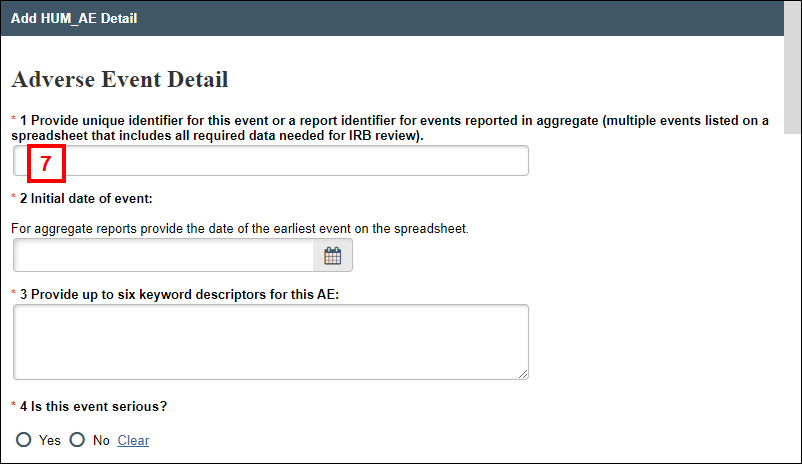
- Answer the remaining questions, then click Continue.
- On the End of Application page, click Save & Exit which then opens the Incident Report workspace, OR
Click Submit to open the submit activity, then go to step 11.
Notes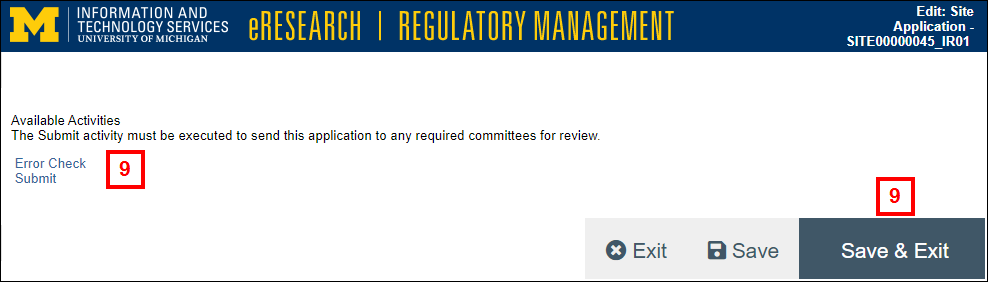
- The system validates that all required fields are complete. Any errors must be resolved before the IR can be submitted.
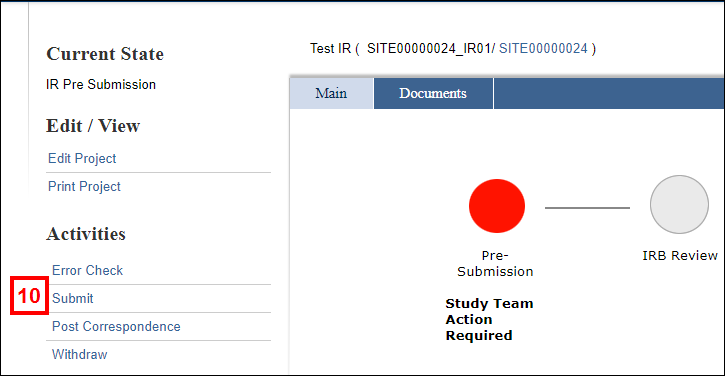
- The Incident Report is in the state of IR Pre Submission.
- To view an Incident Report that is in progress, click Edit Project from the Incident Report workspace.
Submit an Incident Report
Only the PI can submit an application’s Incident Report.
- Click the Submit activity from the Incident Report workspace.
- Read the Investigator Assurances, then check the box to indicate that you agree.
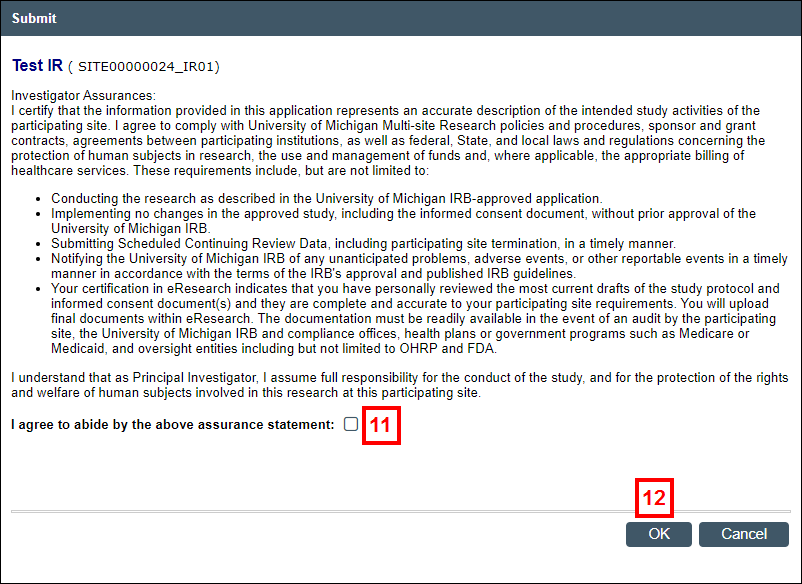
- Click OK to submit the Incident Report for review by the appropriate committee(s).
Notes- The state of the Incident Report changes to IRB Review.
- Once submitted, an AE/ORIO can no longer be edited unless reviewers require changes.
- You can open the Incident Report later from the AE/ORIO tab of the Participating Site workspace.
