Overview
A Termination Report is used only for terminating an approved participating site application upon completion of the research or for other reasons, such as low accrual, toxicity, funding, etc. Once the report is finalized, the site application will be terminated and archived. Once archived, you cannot modify or use the site application for research going forward.
Important Only one Termination Report or Continuing Review for a site application can be in progress at a time.
Navigation
Role: PI/Study Team Member > My Home
Step-by-Step Process
- Click the Participating Sites or Approved tab on your Home page.
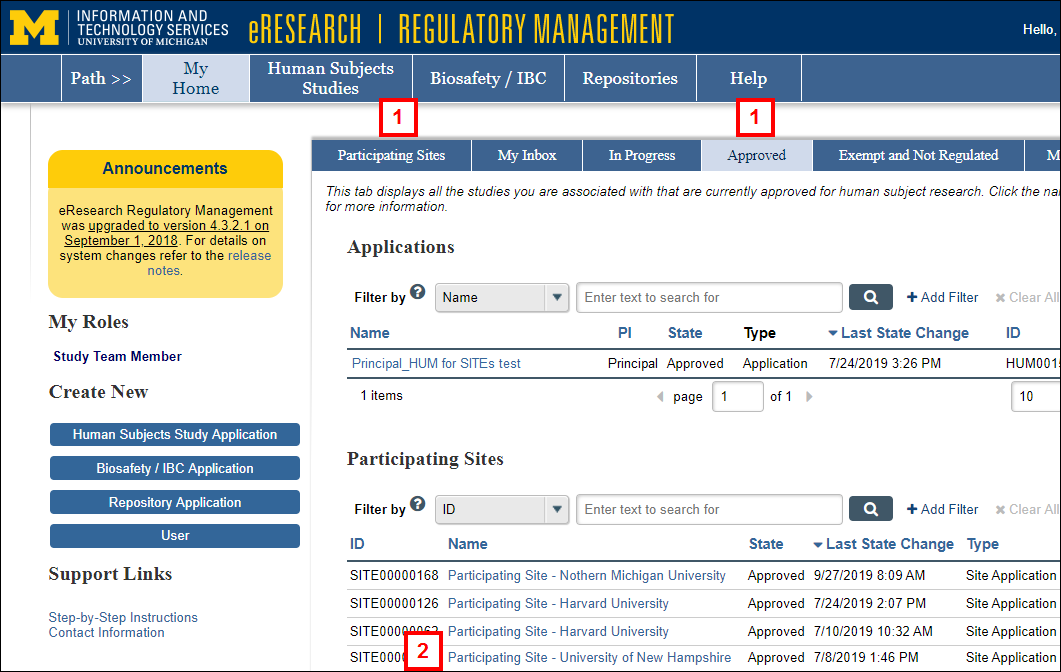
- Click the Name of the Participating Site Application.
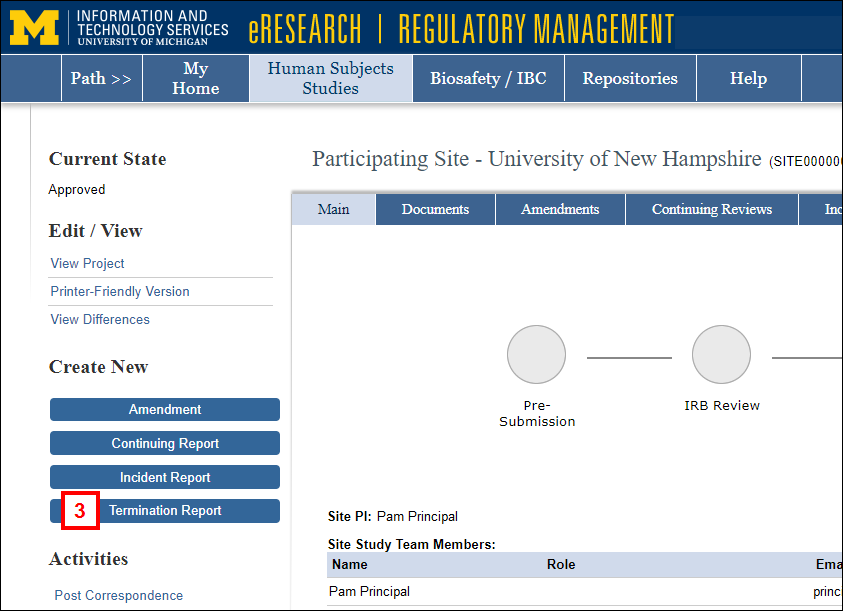
Note The State of the application must be Approved. - Click the Termination Report button under Create New.
- Select the applicable Termination Reason.
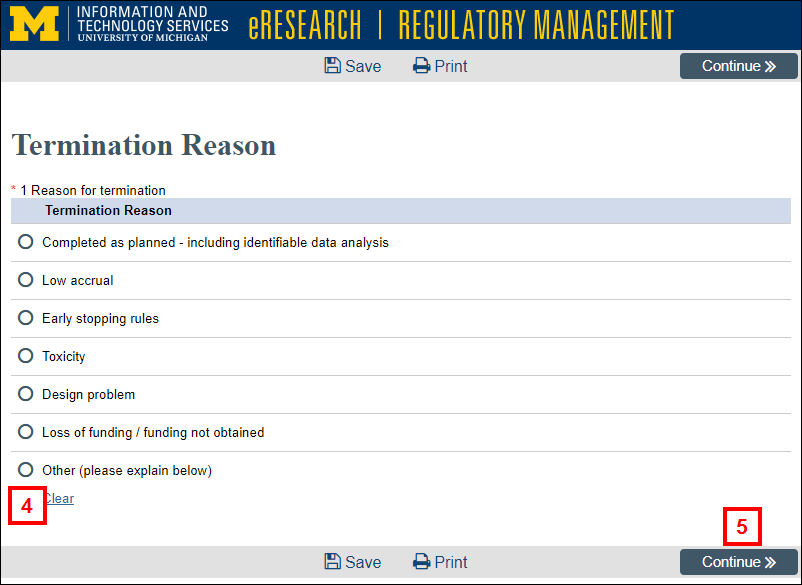
- Click Continue.
- Complete the required fields on the Enrollment Information page.
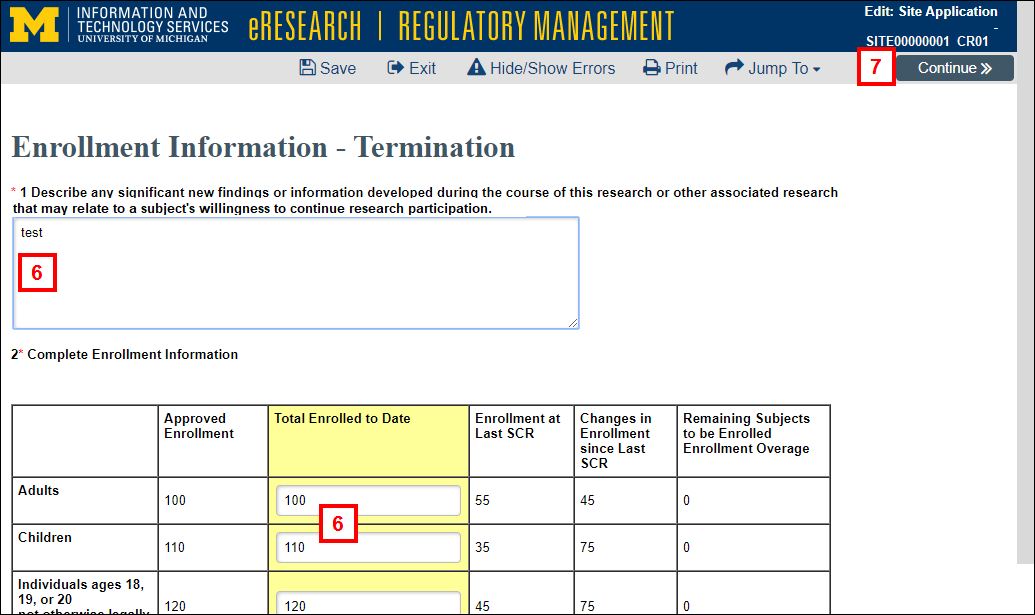
- Click Continue.
- Click Submit on the End of Application page.
Notes- If you are not ready to submit, click Save & Exit. You will be returned to the Participating Site Termination workspace. From there, click the Submit activity to submit at a later time.
- The system validates that all required fields are complete. Any errors must be resolved before the Termination Report can be submitted.
- Read the Investigator Assurances, and check the box to indicate that you agree.
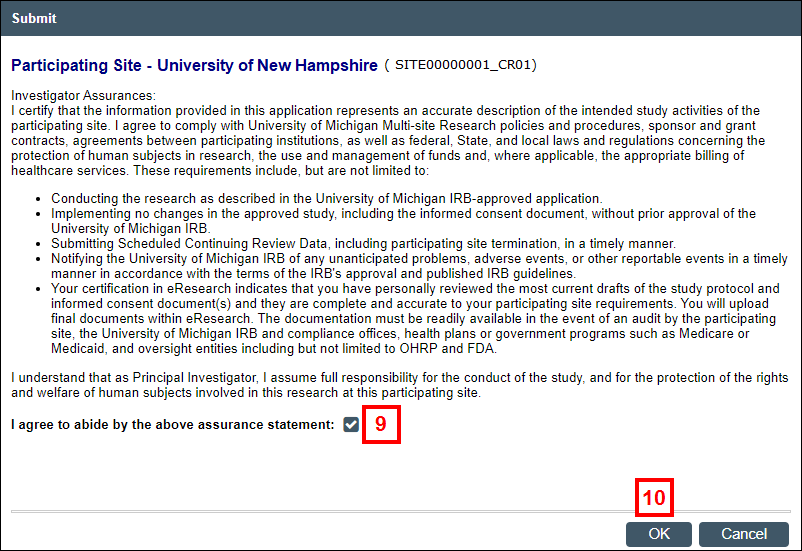
- Click OK.
Notes
- The State of the termination changes from SCR Pre Submission to IRB Review.
- Track the progress of a termination on the In Progress tab of your Home page.
Last Updated
Thursday, October 24, 2019
