Overview
Although it is not required, a committee member can log in to eResearch Regulatory Management (eRRM) before a committee meeting to view agenda items.
Navigation
Role: Committee Member > Home Workspace > Meetings tab
Step-by-Step Process
- Click the Committee Member role or verify it is selected on the Home Workspace.
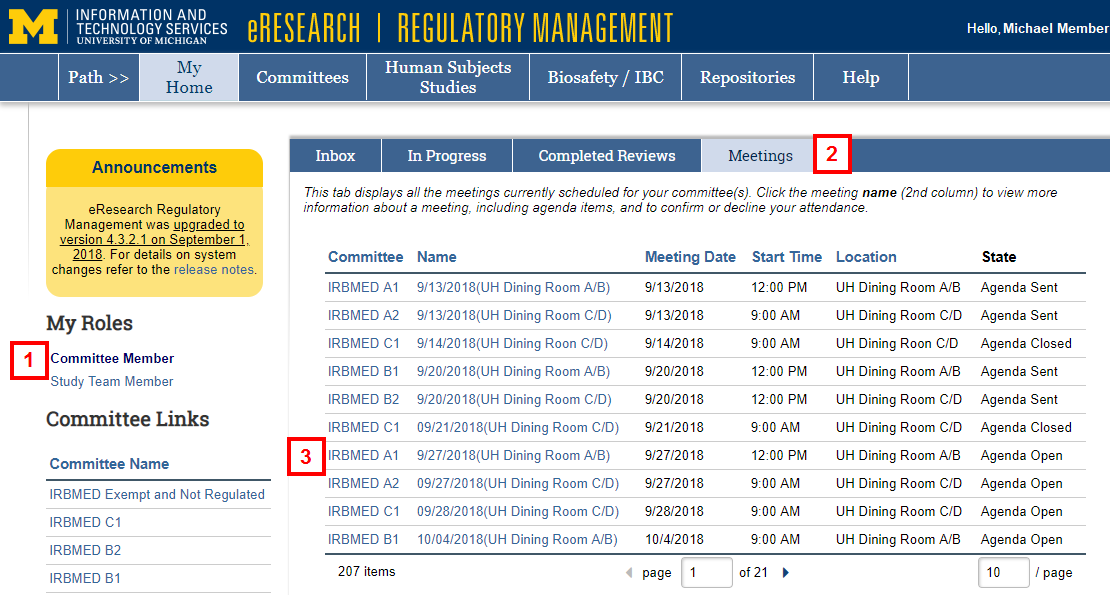
- Click the Meetings tab.
- Click the Name of the meeting date.
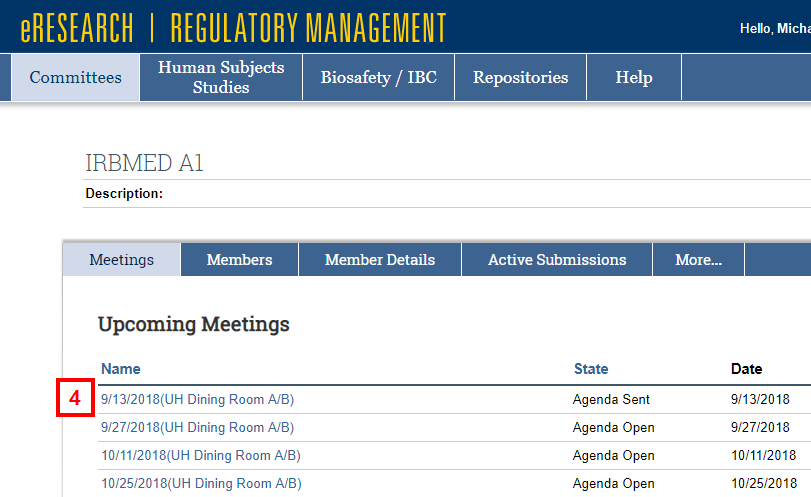
- Click the Name of the meeting date under Upcoming Meetings.
- Click View Agenda by Reviewer.
Note The agenda can also be viewed by clicking on View Agenda by Submission Type.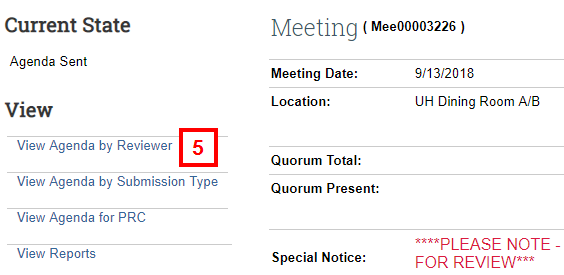
The Meeting Agenda opens in a separate window. It displays the Meeting Date, Start Time, Location and the following:
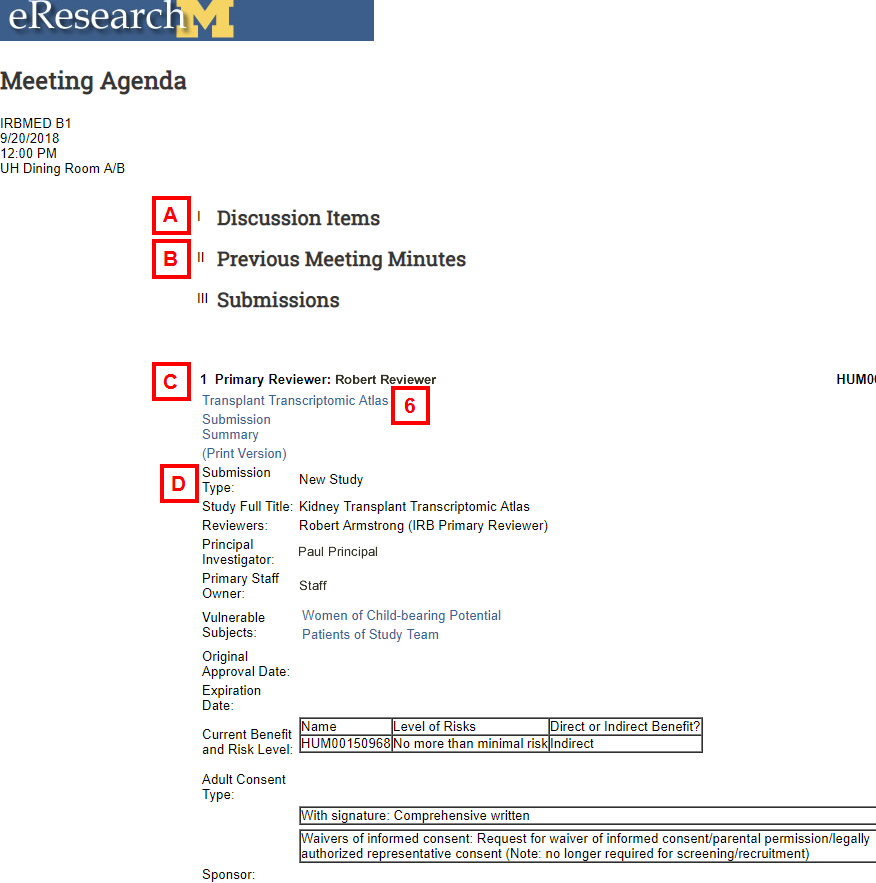
- Discussion Items that have been added to the agenda.
- Previous Meeting Minutes that have been added to the agenda.
- Primary Reviewer for each of the submissions.
Note If viewing agenda by Submission Type, the type of Submission will display in this area. - Submission Information:
- Submission Summary opens a Submission Summary for the submission.
- Print Version opens a Print view of the Submission.
- Reviewers Primary Reviewer and also any Secondary Reviewers assigned to the submission.
- Principal Investigator name of the PI for the submission.
- Expiration Date of the submission.
- Current Benefit and Risk Level of the study.
- If desired, click the Name of the submission to open the submission for review in a separate window.
- Click View Study, Printer Friendly Version or Submission Summary from the Edit/View menu to view the study information.
Note View Study will change depending on the type of submission being viewed (e.g., View Amendment, View Adverse Event, etc.).
Last Updated
Tuesday, October 15, 2019
