Overview
When you log in to eResearch Regulatory Management (eRRM), your Home Workspace displays. You can initiate a variety of activities, access other workspaces, and view any items that require your attention.
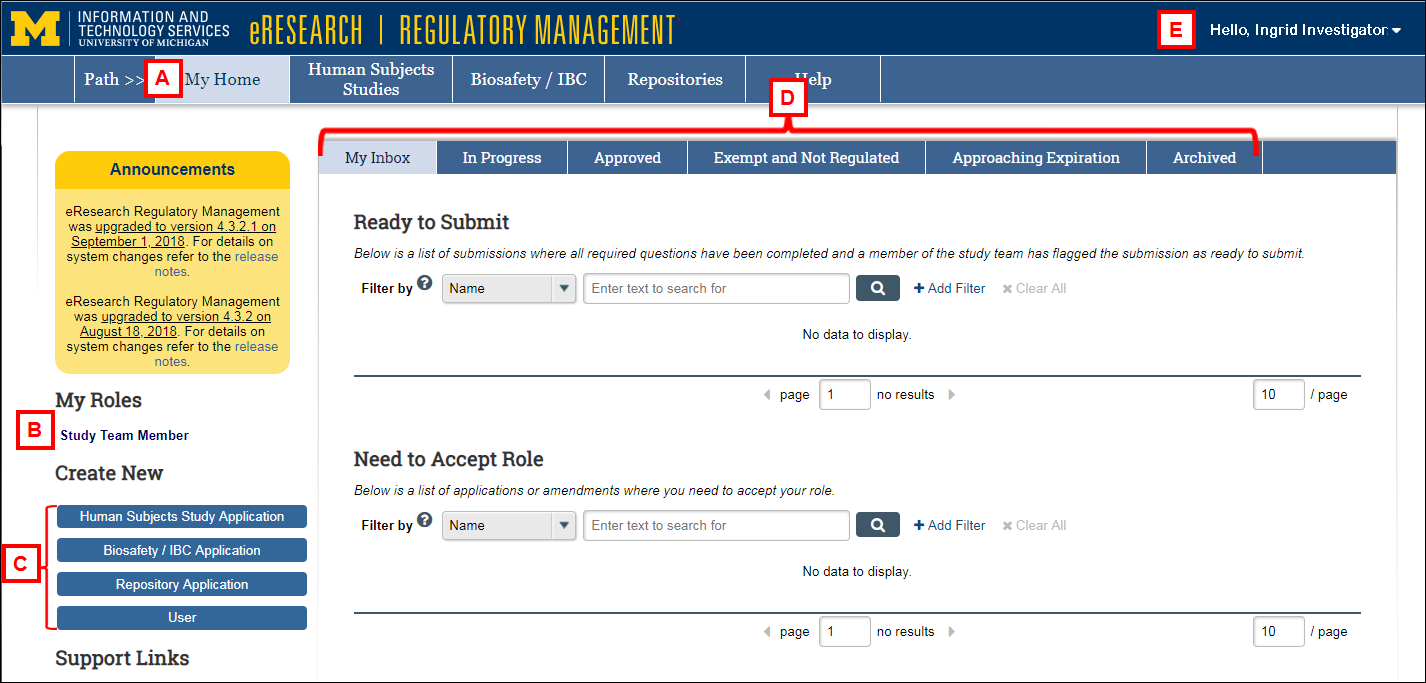
| Letter | Description | |
| A | My Home | Click to return to your Home Workspace from any eRRM page. |
| B | My Roles | Displays all available system roles assigned to you. Your current system role displays in bold text. Click the link(s) to switch roles, if applicable. |
| C | Create New |
Click the applicable option to create a new: Human Subjects Study Application Biosafety/IBC Application Repository Application User. Click to create a new eResearch User Account. |
| D | Workspace Tabs |
My Inbox. Contains Items that are ready for submission, require you to accept your role, and/or require actions by the study team. In Progress. Study applications you are associated with that have not yet been approved. Approved. Approved studies you are associated with. Exempt and Not Regulated. Exempt and non-regulated studies that you are associated with. Approaching Expiration. Approved studies and registrations that are expiring within 90 days. Archived. Studies you are associated with that have been terminated, disapproved, withdrawn, or expired. |
| E | delete | Click to Logoff from eRRM. |
