Overview
Study teams receive an email reminder that their research application is due for a Scheduled Continuing Review (SCR) 30, 60, and 90 days prior to the study expiration date. Create an SCR to renew or terminate your study.
Navigation
Role: PI/Study Team Member > Home Workspace
Step-by-Step Process
- Click the Approved tab.
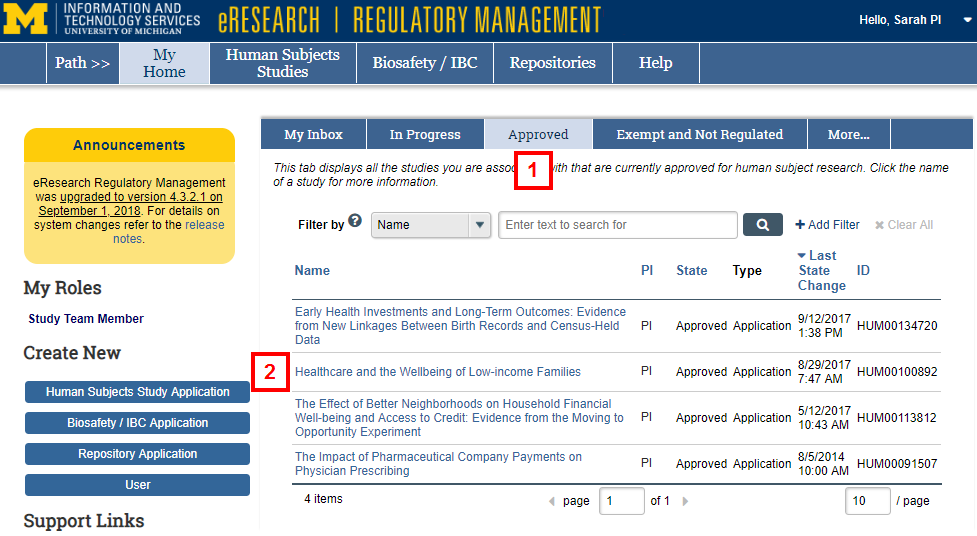
- Click the Name of the study.
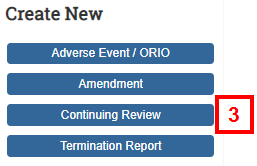
- Click Continuing Review on the Study Workspace.
- Enter or verify the SCR Title.
Note If you edit the title, include the HUM # of the original study to make tracking easier.
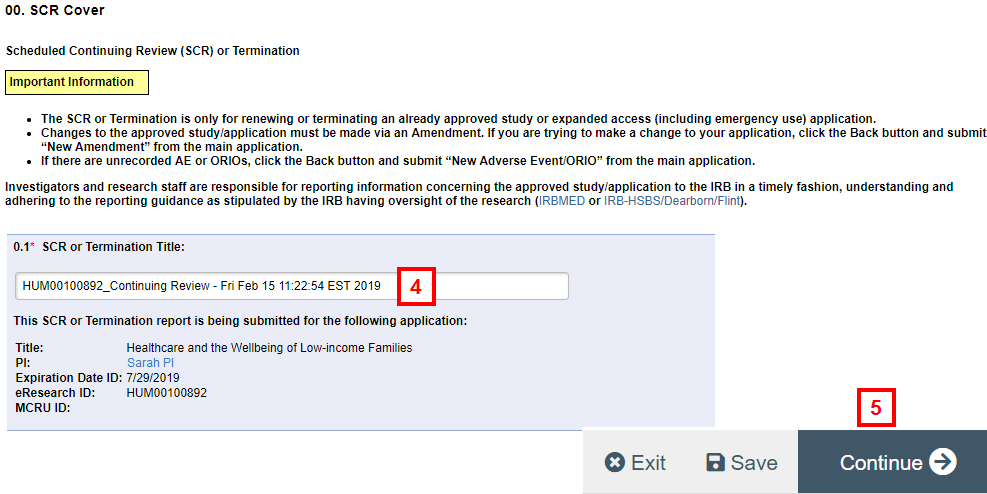
- Click Continue.
- Complete the required information on each page of the Continuing Report.
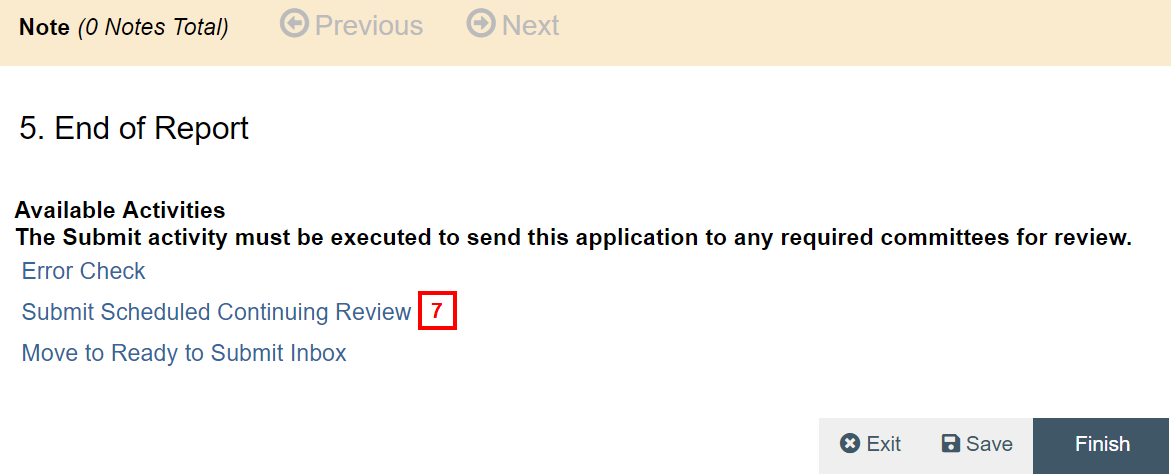
- Click Submit Scheduled Continuing Review.
Notes- If applicable, run an Error Check and correct any errors before submitting the SCR.
- Click Move to Ready to Submit Inbox if you are not ready to submit yet. The Ready to Submit Inbox is located under the My Inbox tab of your Home Workspace.
- Click OK.
Important By clicking OK, you will no longer be able to modify the application. You will be notified of the review result by email.
Track the progress of a continuing review on the In Progress tab of your Home Workspace. Once the continuing review is approved, go to the Continuing Reviews tab in the Study Workspace to view the Continuing Review.
Last Updated
Thursday, February 27, 2025
