Overview
Study teams are required to submit Adverse Events (AEs) and Other Reportable Information and Occurrences (ORIOs) via eResearch Regulatory Management (eRRM). AE/ORIO reports can also be created to follow-up on previously reported AEs/ORIOs.
AEs include any experience or abnormal finding that is harmful or increases the risk of harm to research participants. ORIOs include audits, other reports, protocol deviations, protocol violations, facility/data accidents, and complaints (which includes lapses in approval). See the eRRM Glossary for more details.
The basic steps for initiating an AE/ORIO report are the same regardless of the type of AE/ORIO being reported; however, the report form that opens and the information you are asked to provide will depend on the type of AE/ORIO indicated. The following procedure acts as a general guide for reporting an AE/ORIO of any type.
Navigation
Role: PI/Study Team Member > Home Workspace
Step-by-Step Process
- Click the Approved tab.
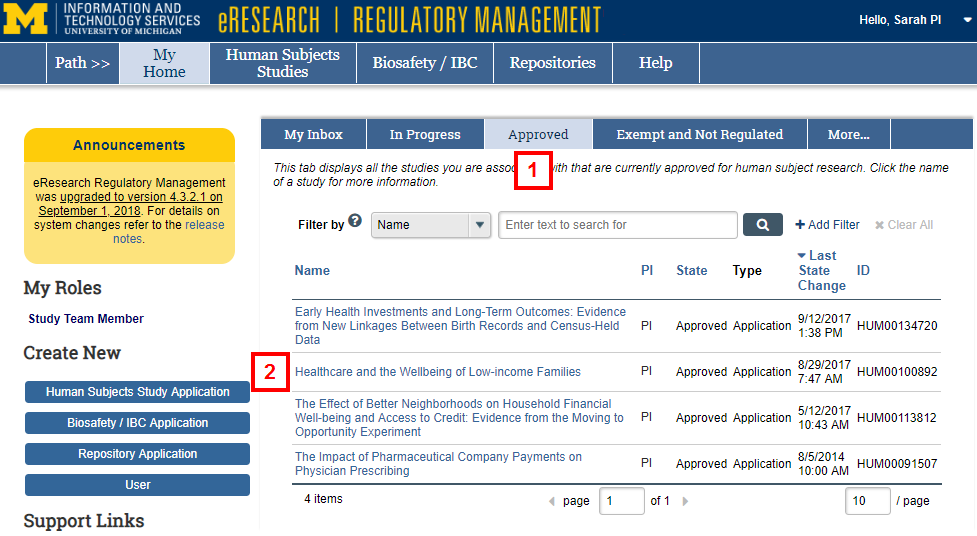
- Click the Name of the study to view the Study Workspace.
- Click Adverse Event/ORIO.

- Choose the Type of Report radio button.
Note If ORIO is selected, answer the additional questions that display.
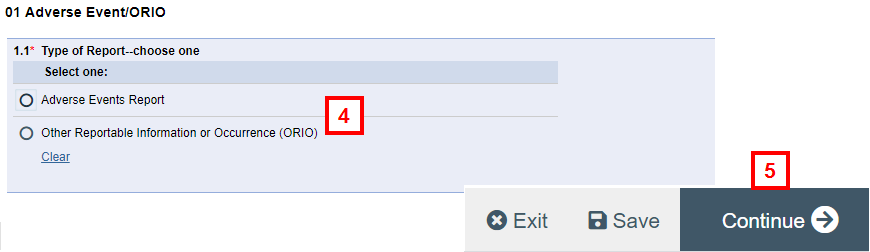
- Click Continue.
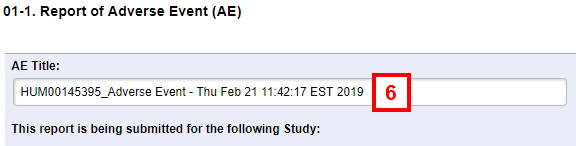
- Enter or verify the Title.
Note A default title is created and includes the HUM # of the original study, the type of report (i.e., AE or ORIO), and the date/time it was created. If you edit the default, keep the HUM # and type of report in the title to make tracking easier.
- Click the applicable Adverse Event or ORIO type radio button.

- Click the applicable radio button to indicate if the report includes follow-up to previously reported events.
Notes
- If you select Yes, you will be required to indicate the previous reported events.
- Only previously finalized AEs (or ORIOs) of the same type are available to be chosen.
- Choosing a prior AE (or ORIO) will pre-populate the current submission with information from the prior submission (question 2.2 AE detail for AEs, the entire relevant ORIO page for ORIOs). Information previously entered in the current submission may be overwritten.
- Click Continue.
- Depending on the type of AE or ORIO you are reporting, the applicable report form will open. Complete the required information on each page, and click Continue to save and go to the next page.
- If you are not yet ready to submit the AE/ORIO, click Finish to save and exit the report.
Note You will be taken to the AE/ORIO Workspace from where you can edit, submit, or withdraw the report.
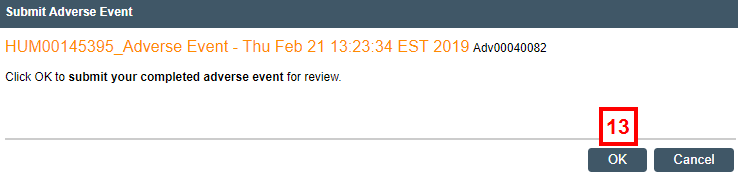
- If you are ready to submit the report for review, click Submit Adverse Event (or Submit ORIO, if applicable).
Notes- The system automatically validates all required fields are complete.
- Any returned errors must be addressed before continuing.
- Click OK.
Notes- Once submitted, an AE/ORIO can no longer be edited unless reviewers require changes.
- A message indicating AE/ORIO(s) in Progress displays in the study workspace.