Overview
Amendments are created for approved protocols when any part of the protocol requires a change. Scientific and PI Amendments will have a "coversheet" in the form of an AME Summary. An amendment summary will describe what is changing and why to provide content for future reports and guidance to the Reviewers.
Notes
- A scientific amendment allows the data on the following pages to be changed: General Information, Species, Location Information, Procedures, Procedure Summary, Adverse Consequences, Use Justification and Alternatives.
- A change PI amendment allows the data on all application pages to be changed.
There are four activities or four opportunities in which to create an amendment summary. Each activity contains the same AME Summary questions.
- Maintain AME Summary - by Protocol Editors, PI, and the ACUO
- Notify PI that Amendment is Ready to Submit - by Protocol Editors
- Submit Amendment (in Amendment Pre-Submission state) - by PI
- Submit Changes - by PI
Step-by-Step Process
Maintain AME Summary
This activity is available to the PI and Protocol Editors on amendments that are in the states of AME Pre-Submission, AME PI Changes Pre-Review, or AME PI Changes Committee Review. The activity is is available to the ACUO RCA and Office roles in any state.
- From the Amendment workspace, click the Maintain AME Summary activity.
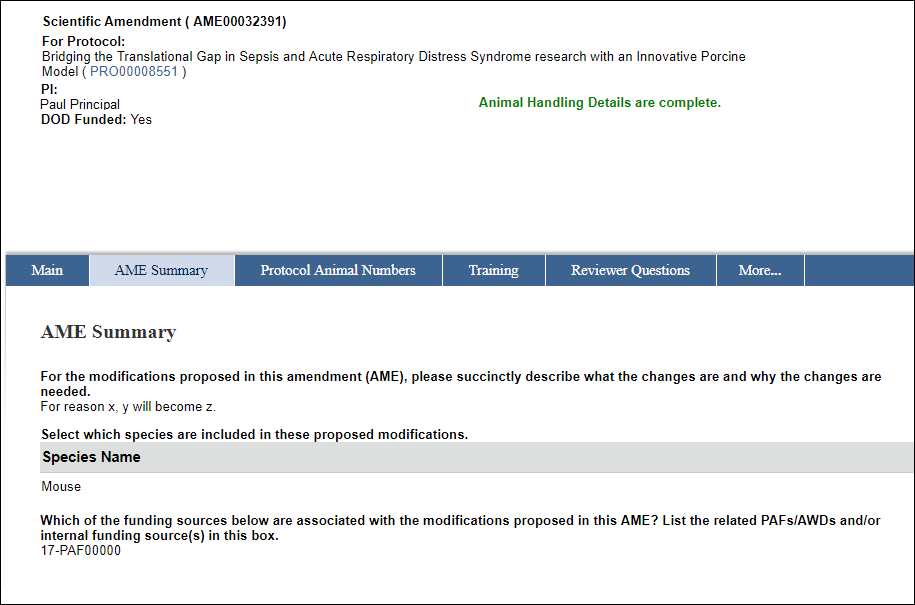
- Enter a description in the For the modifications proposed in this amendment (AME), please succinctly describe what the changes are and why the changes are needed field.
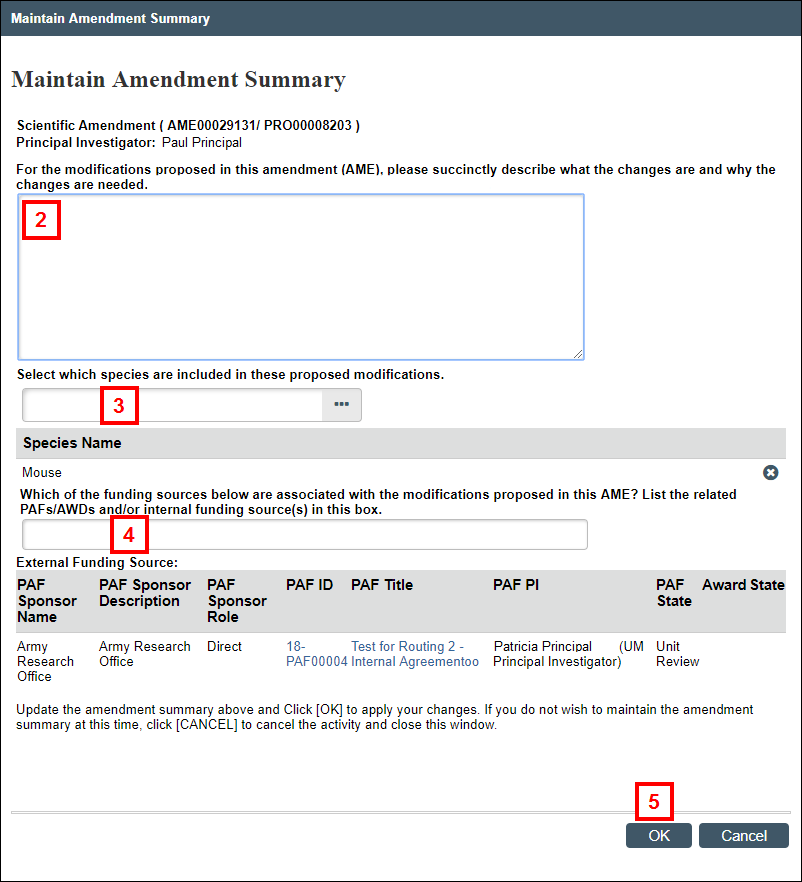
- Enter or select the species.
- Enter the associated PAFs/AWDs and/or internal funding source(s), if applicable.
Note This question will only display for DoD funded protocols. - Click OK.
Notify PI that Amendment is Ready to Submit
This activity is available to Protocol Editors on amendments that are in the states of AME Pre-Submission, AME PI Changes Pre-Review, or AME PI Changes Committee Review. It contains the same questions as in the Maintain AME Summary activity, with an additional question for entering comments.
- From the Amendment workspace, click the Notify PI that Amendment Ready to Submit activity.
Note Error checking is performed on the amendment when it is submitted. If any errors are found, a list displays. All errors must be resolved before the amendment can be successfully submitted. - Enter Comments.
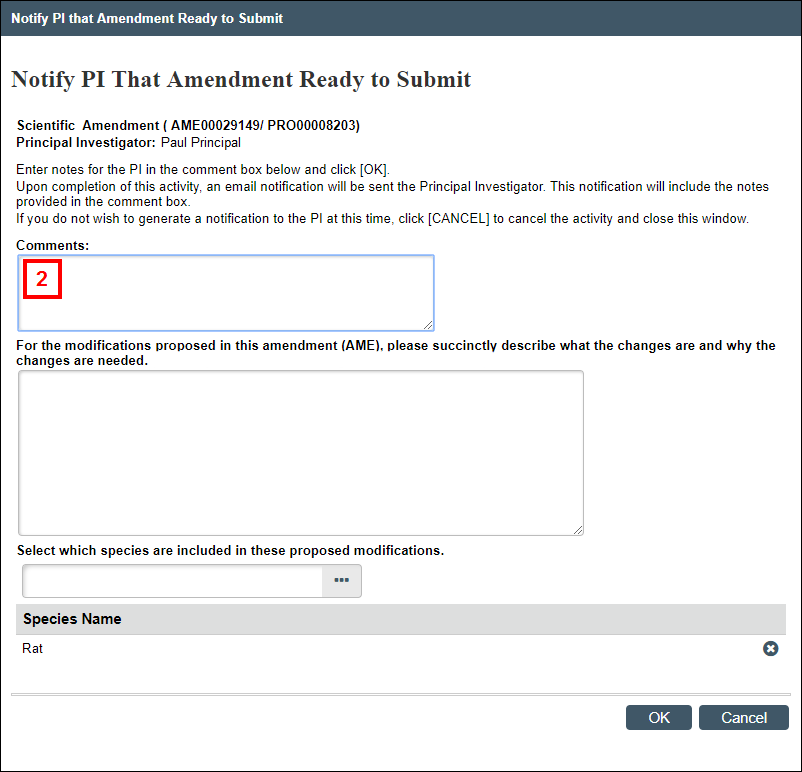
- Answer the remaining questions (see steps 2-5 above in the Maintain AME Summary section), as applicable.
The PI will be notified that the Amendment is ready to be submitted, and the Amendment will include the summary entered by the Protocol Editor.
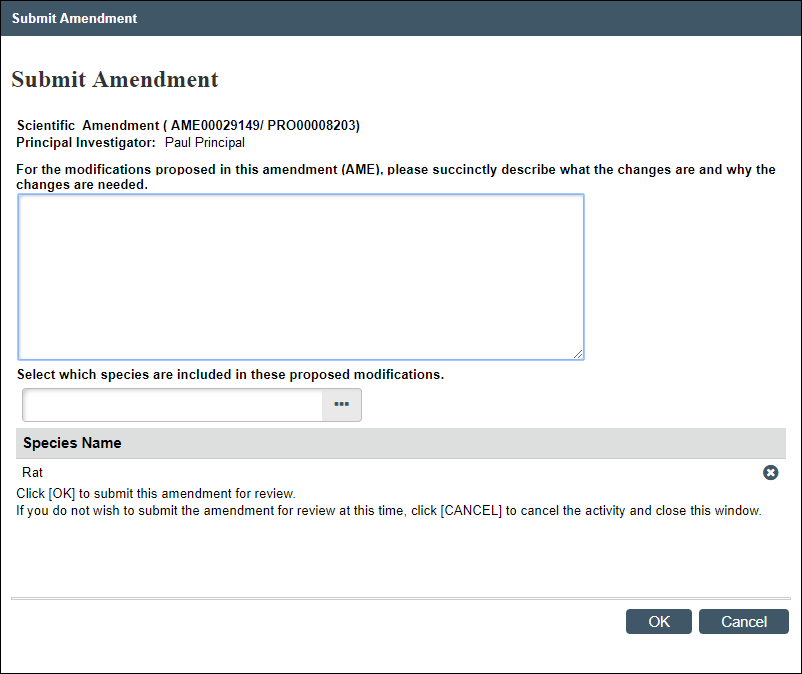
Submit Amendment
This activity is available to the PI on amendments that are in the states of AME Pre-Submission, AME PI Changes Pre-Review, or AME PI Changes Committee Review.
- From the Amendment workspace, click the Submit Amendment activity.
Note Error checking is performed on the amendment when it is submitted. If any errors are found, a list displays. All errors must be resolved before the amendment can be successfully submitted. - Answer the remaining questions (see steps 2-5 above in the Maintain AME Summary section), as applicable.
Submit Changes
This activity is available to the PI on amendments that are in the states of AME Pre-Submission, AME PI Changes Pre-Review, or AME PI Changes Committee Review.
- From the Amendment workspace, click the Submit Changes activity.
Note Error checking is performed on the amendment when it is submitted. If any errors are found, a list displays. All errors must be resolved before the amendment can be successfully submitted. - Enter Optional Note to Reviewers.
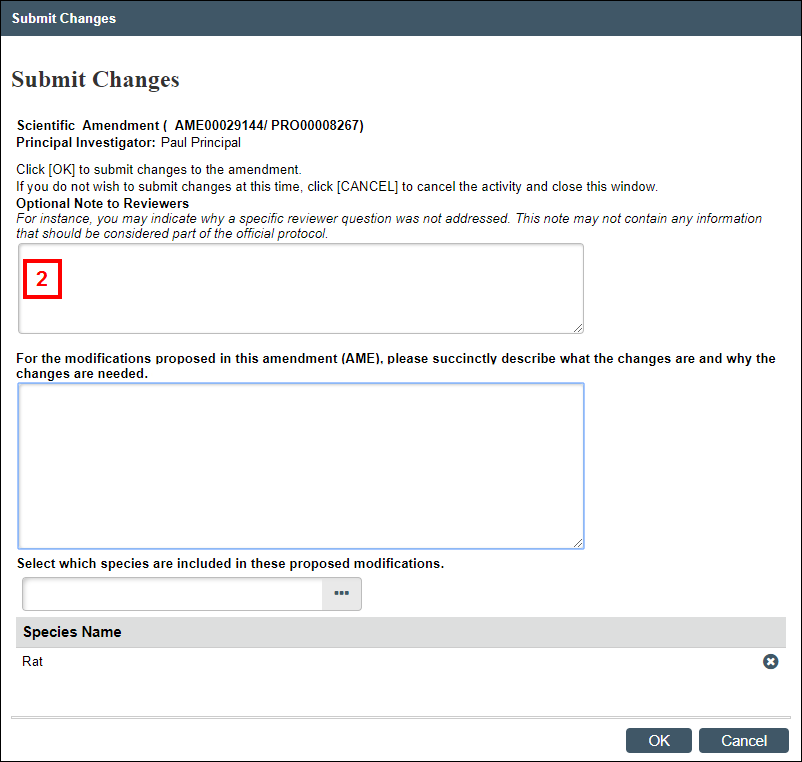
- Answer the remaining questions (see steps 2-5 above in the Maintain AME Summary section), as applicable.
Viewing the Summary After any Submission method
Once submitted, the AME Summary tab on the Amendment Workspace will be updated with the information entered.
The Amendments tab on the Protocol Workspace will list Summary and Involved Funding Source, as applicable.
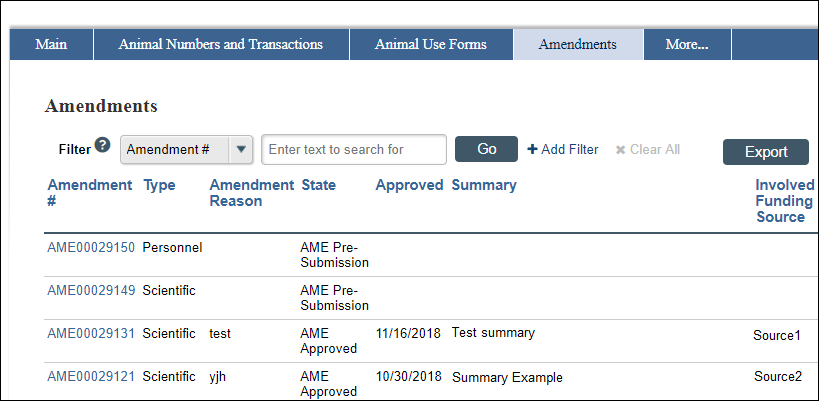
Note Once entered and submitted, the AME Summary information remains in place; in other words, if an Amendment workspace activity is re-run, the amendment summary information is already filled out and can be edited, if needed.
